© 2025 Syngene International Limited. All rights reserved

Speed and Efficiency
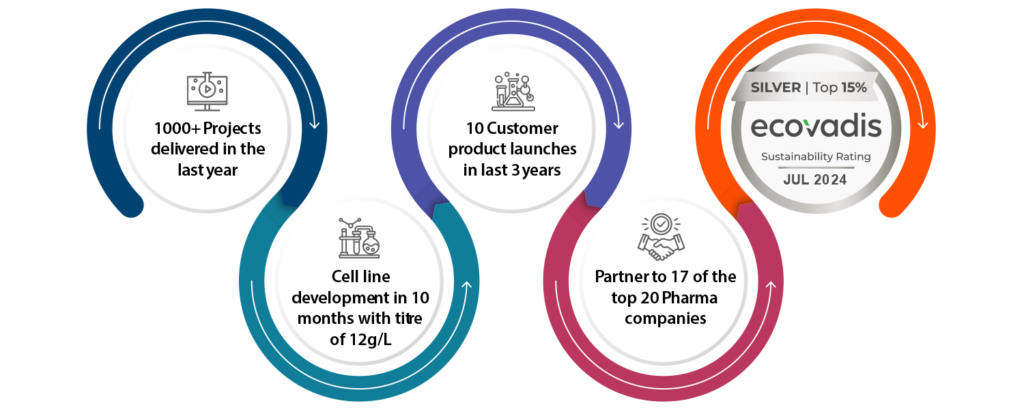
Syngene delivers fast, reliable results. Our streamlined processes reduce costs, accelerate timelines, and ensure seamless project execution, helping bring your therapy to market faster.

End-to-End Capabilities
From drug substance & drug product development to manufacturing, Syngene offers comprehensive, in-house services across modalities in small molecule, biologics, oligonucleotides. As your single partner, we ensure smooth transitions and faster time-to-market, simplifying the process and enhancing efficiency.
Scalability in Commercial Manufacturing
Our cGMP-compliant facilities support scalable production of APIs and drug products, ensuring quality and global compliance. We handle both small- and large-scale production, offering flexibility to meet market demands, with notable projects including mAb and API manufacturing at USFDA-approved facilities.
Meet our experts

Alex Del Priore
Sr. Vice President – Manufacturing Services

Jeffrey Wong
Vice President Sales, Biologics

Manoj Babu
Vice President Sales, Small Molecules Development & Manufacturing

Julien Hugon
Vice President , Small Molecules Development and Manufacturing



