Eliminating Early‑Stage Bottlenecks in Biologics Development
The mAb Accelerator program is created in response to a fundamental disconnect we repeatedly saw in early and mid‑stage biologics development i.e. innovation is moving faster than the systems supporting it. Across biotech and biopharma, we observed strong science being delayed, not because of technical limitations but because of organizational uncertainty, funding cycles, governance approvals, partner alignment, and portfolio decisions. These delays often occur before the most value‑creating work cell line development could even begin.
To address these challenges, Syngene introduces the mAb Accelerator program, which initiates cell line development immediately, so your molecule progresses while you secure approvals and funding. The program then advances your asset from Gene‑to‑GMP typically in 9 months (with DP ~10 months), but expedited timelines are available, through an integrated end‑to‑end CDMO footprint across the U.S. and India. It is powered by SynWeave™ high‑titer CLD platform (7–12 g/L), FDA/EMA‑approved sites, and state‑of‑the‑art 2 KL and 4 KL single‑use bioreactors, totaling 50 KL of global single‑use capacity.
3 Core Problems mAb Accelerator Solves for Biopharma Companies
mAb Accelerator is designed to help you accelerate innovation by providing the lowest risk path to IND. It democratizes access to proven transposase-based cell line development, ensuring that scientific progress is driven by data and informed decisions rather than capital constraints.
Core Problems | Syngene Solution |
|---|---|
a) Time Lost to Inaction • Cell Line Development (CLD) is often delayed while approvals, funding, or partnerships are finalized • These months create zero asset value, even though the program clock keeps ticking | a) Head Start That Actually Changes Outcomes By starting CLD immediately, customers gain up to a 4 month head start that translates into
|
b) Concentrated Early Stage Risk • The highest technical and financial risk exists at the very beginning (CLD can represent 10-30% of pre-IND spending) • Traditional CDMO models shift this risk almost entirely onto the customer, with full upfront commitments | b) Risk Sharing by Design This is not acceleration through speed alone; it is acceleration through shared conviction. Customers move forward before certainty sets in, reducing timeline risk and increasing asset readiness.
• Customers gain progress without front loading exposure • The program signals confidence in the molecule, not just capacity availability |
c) Runway Pressure • Biotechs in particular face capital constraints that force them to choose between advancing science and preserving cash • This trade off slows progress and weakens negotiating leverage with investors or partners | c) Capital Sensitive, Flexible Commercial Models The program is structured around the biotech economics so that customers can preserve runway while still making real scientific progress.
• Spend aligned to meaningful development milestones • Reduced upfront financial pressure during the highest risk phase |
Distinctive Features of the mAb Accelerator Program
Molecule First.
Head‑Start Model
Rapid contracting through simplified templates, enabling Cell Line Development (CLD) to begin within 4 weeks of signature, saving up to ~4 months while approvals or funding progress in parallel.

Risk‑Sharing Approach
Syngene commits capacity, resources, and scientific expertise upfront, sharing early technical and operational risk alongside the customer.

Flexible Commercial Models
Designed around biotech economics to preserve runway, with milestone‑based spend, shared success models, and reduced upfront financial exposure during early development.
Integrated Programs. Accelerated Timelines.

Accelerated Time to Clinic
Seamless progression from Gene to GMP in ~10 months, enabled by integrated drug substance (DS) and drug product (DP) capabilities.

Proven CLD Platform
Powered by SynWeave™, delivering 7–12 g/L titers with robust scalability.

End‑to‑End ADC Continuum
Integrated mAb development and GMP bioconjugation under one roof, reducing supply chain complexity and timelines.

High‑Throughput Fill‑Finish
Clinical and commercial DP capabilities supporting up to 1 million vials per day.
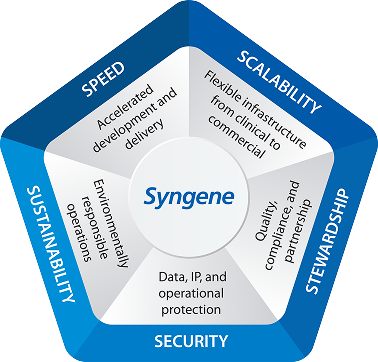
Scale. Compliance. Reliability.

Global, Integrated Footprint
U.S. and India manufacturing network with 50 KL single‑use bioreactor (SUB) capacity, including flexible scale‑up using 2 KL and 4 KL SUBs.

Demonstrated Execution Excellence
150+ biologics programs | 25+ INDs | 250+ GMP batches delivered.

Strong Regulatory Pedigree
Multiple FDA and EMA‑approved sites, supporting global clinical and commercial pathways.

Sustainable Operations
Manufacturing powered by >80% green energy, supporting responsible and resilient supply chains.
A Partnership That Takes Your Molecule All the Way
Our Gene to GMP program is more than a service, it’s a strategic alliance that aligns our capabilities with your goals. By supporting your cell line development program, we’re not just reducing your financial risk, we’re demonstrating our confidence in your molecule’s potential. Whether you’re a biotech startup or a global pharma leader, Syngene is committed to being your partner from bench to bedside.
Let’s take the first step together, because your success is our starting point.
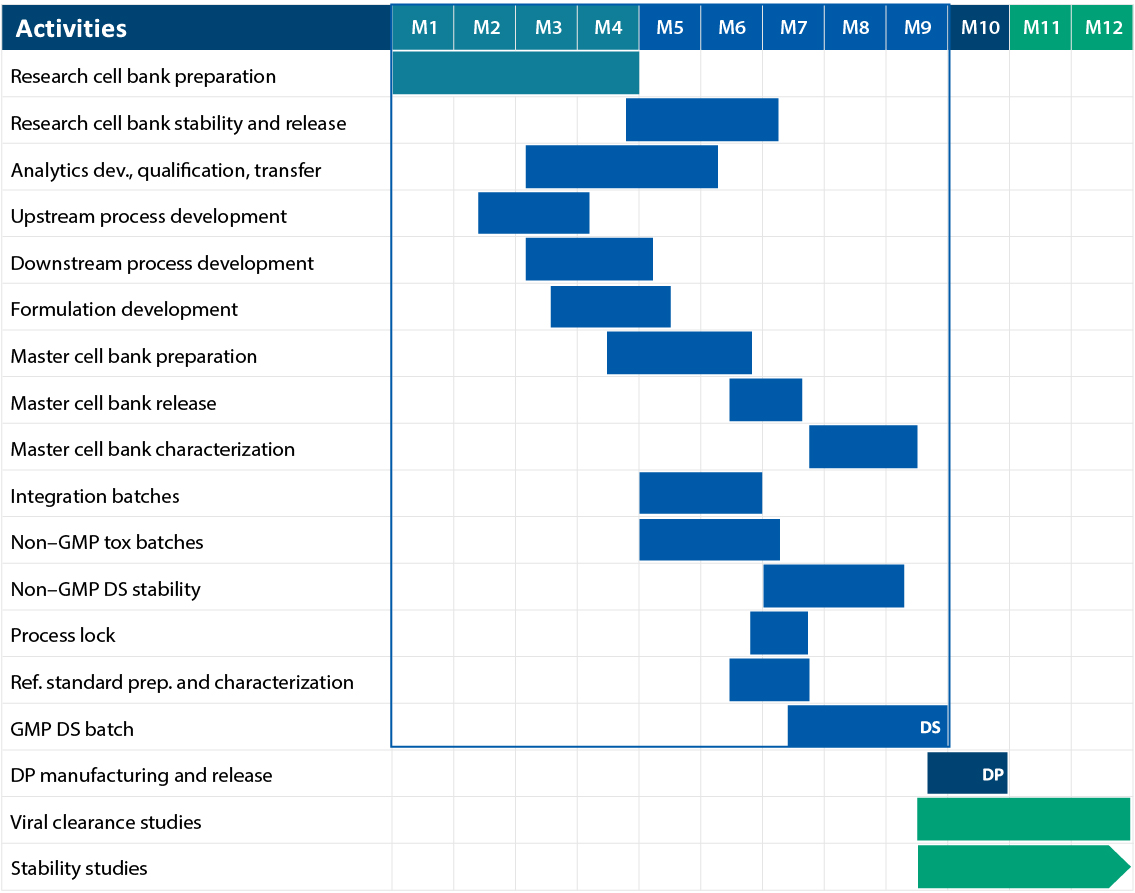
Gene to GMP in 9 Months
Powering Progress from Molecule to Market
At Syngene, we empower biopharmaceutical companies to bring large molecule therapies to life through our state-of-the-art biologics CDMO services across the U.S. and India. With over 30 years of experience, we deliver integrated, expert-driven solutions that accelerate development, ensure compliance, and turn breakthrough ideas into market-ready products.
Fast track your IND approval with Syngene

Why Syngene Biologics
150+
Successful projects
25+
INDs enabled
250+
GMP batches
50 KL
Global GMP* capacity
2 KL & 4 KL
SUBs across the U.S. and India
7-12 g/L
Titer with SynWeave Platform
>80%
Green power
1M
Vials per day
Target ID, Validation and Lead Generation
Target ID, Validation and Lead Generation
Target ID, Validation and Lead Generation
Target ID, Validation and Lead Generation
Target ID, Validation and Lead Generation
Target ID, Validation and Lead Generation
Target ID, Validation and Lead Generation

Hit to Lead
Our Discovery Services provide drug candidates that have been screened and validated through our pre-clinical trials and humanized models
Gene to GMP
Our SynWeave™*
high-yielding cell line platform consistently provides 7-12 g/L titers using our process development capabilities in 10 months
Launch Ready
Our USFDA and EMA approved GMP facilities feature single-use bioreactors with capacities of 2,000 L and 4,000 L totaling 50,000 L designed to deliver economies of scale
Early Adopter
We are on the move with next generation technologies that reduce cost ($/g) by 50% using perfusion and continuous purification processing
Our biologics offerings across the spectrum (DS and DP)
Mammalian
- 500 L, 2,000 L and 4,000 L Single-use bioreactors
- Dedicated suites for multi-product handling
- Downstream Harvest (centrifuge or depth filtration)
- GLP-certified virus testing and clearance
USFDA, EMA, MHRA approved facilities
Microbial
- 500 L, 2,000 L and 4,000 L Single-use bioreactors
- Continuous centrifugation
- 60 cm Chromatography
- Automated Tangential Flow Filtration (TFF)
Plasmid DNA & mRNA production system
DP Formulation
- Multi-product clinical line (vials / PFS¹)
- 1 million vials per day
- 12-50L Batch size
- Vial filling size (0.5ml – 10 ml)
- PFS filling size (0.3 – 1 ml)
Manual visual inspection, semi-auto labeling, manual packing
cGMP manufacturing facilities across the U.S. and India

Bayview, Maryland, U.S.
Mammalian Manufacturing
16 KL / 28 KL

Mangalore, India
SM CDMO API manufacturing
~70 KL (expandable)

Biocon Park , Bangalore, India
Mammalian, SM API, DP OSD
LM CDMO: 8 KL
SM CDMO: ~70 KL

Unit 3, Bangalore, India
Mammalian, DP-1/2
LM CDMO: 10 KL / 28KL
DP: Vials, 2 lines, 500 vials/min
Get in touch
Sign-up for our newsletter
Get in touch
Other links
© 2025. Syngene International Limited