Challenges in Manual Liver Microsomal Stability Assays for ADME Studies
In the drug discovery process, Pharmacokinetics is one of the critical research areas that evaluates the absorption, distribution, metabolism, and excretion (ADME) of new chemical entities (NCEs), aiding in the selection and optimization of candidates for advancement toward a new drug application. Metabolism is a key area in ADME screening, where thousands (5,000−20,000) of NCEs are screened, typically through assays such as the in vitro liver microsomal stability (LMS) assay, to assess their metabolic stability. Performing the LMS assay manually typically limits the handling of compounds to around 24 per experiment per scientist, thereby prolonging the DMTA cycle (Design→ Make→ Test→ Analyze). The employment of robotics enhances the handling of compounds from 24 to 45 per experiment per scientist.
The LMS assay involves four stages: Experimental preparation, Experimentation, Analysis, and Reporting.
The experimentation process requires highly skilled staff for effective execution because multiple plates are handled simultaneously. Limitations of manual experimentation include high demand, low productivity, and a lower percentage of right-first-time.
The challenges with the LMS assay are:
1) High Demand: An in vitro LMS assay is crucial for drug discovery. A manual workflow cannot meet the current screening requirement of 5,000−10,000 compounds annually.
2) Productivity: Typically, an experienced scientist can screen a maximum of 24 compounds in one experiment per day, thereby affecting the longer evaluation period for Design, Make, Test, and Analyze (DMTA) cycle.
3) Limitation: Manual workflow is labor-intensive, error-prone, and demands well-trained and highly skilled personnel for successful execution.
Automating LMS Assays to Improve DMPK and ADME Screening Efficiency
To overcome the challenges of LMS assay, Syngene DMPK set a target to customize the liquid-handling technology platform for in vitro LMS assay.
It starts with identifying a user-friendly automation instrument to replace manual experimentation of in vitro LMS assay with the aim of enhancing productivity.
Process steps:
1) Identifying and qualifying a suitable vendor
2) Estimating the automation instrument cost
3) Procuring and installing the automation instrument
4) Optimizing the process to replicate manual experimental conditions on the automated platform
5) Conducting a manual–automated comparison to confirm equivalence of results
Validation and Standardization of Automated Liver Microsomal Stability Assays
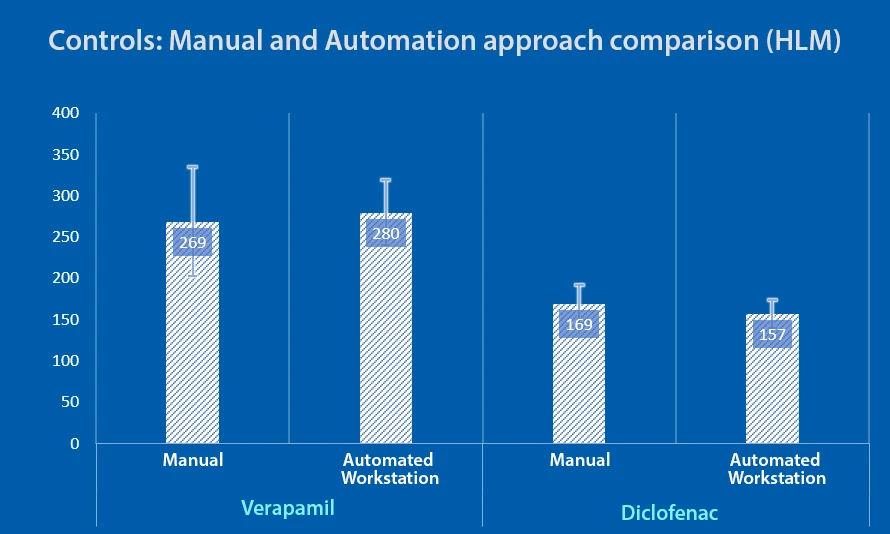
- In vitro LMS assay using human liver microsomes was performed using manual and automated workstation approaches with the same experimental conditions for control compounds – verapamil and diclofenac
- Automated workstation approach exhibited better precision with minimal variation for two assay controls than the manual experiment
Productivity, Quality, and Throughput Gains in ADME and DMPK Workflows
Category | KPI | Unit of measure | Baseline (Year 2023) | Target | Actual (Jan 24 – May 24) |
|---|---|---|---|---|---|
Productivity | Scientist Productivity | Number of compounds tested per scientist per day | 24 | 45 | 45 |
Quality | Right first-time experimentation | % of experiments completed successfully at first attempt | 89% | >95% | 96% |
Deliverable | Capacity enhancement | Average Number of compounds screened monthly | 487 | 833 | 718 |
Scientist productivity increased from 24 to 45 compounds per scientist per day (+87.5% vs 2023 baseline)
Cost analysis indicated substantial financial benefits, with first-year savings of ₹0.68 Cr and projected annual savings of ₹2.05 Cr from the second year onward.
Transforming ADME Studies Through Automated LMS Assays
Syngene’s DMPK team successfully implemented automation for in vitro LMS assays and standardized performance using assay controls (verapamil and Diclofenac). Control data generated through the automated workstation were consistently monitored during routine experiments and remained within established in-house historical ranges of CLint, confirming process reliability.
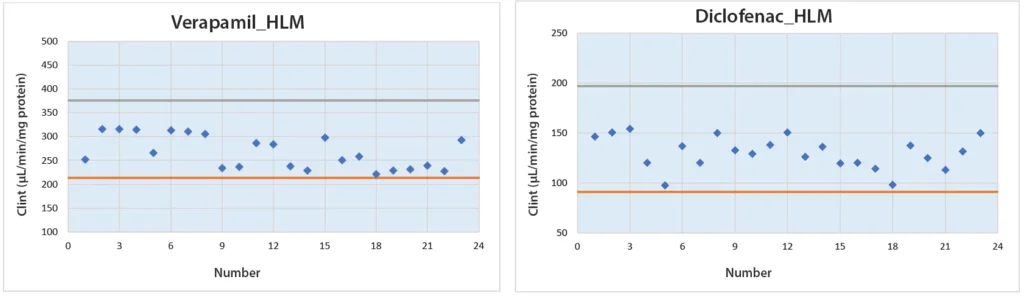
Post-implementation analysis demonstrated measurable improvements in operational efficiency and data quality. The automated approach delivered enhanced productivity, reduced manual variability, and improved overall assay quality, validating the effectiveness of the implemented automation approach.
The KAIZEN initiative was showcased at the 33rd Chapter Convention on Quality Concepts (2024), organized by the Quality Circle Forum of India – Bangalore Chapter, where it earned the Gold Award. The same case study was later presented at the 9th CII National Competition on Digitalization, Robotics & Automation (DRA)–Industry 4.0, securing another Gold Award. These recognitions underscore the strategic importance and tangible benefits of adopting this automation approach.







