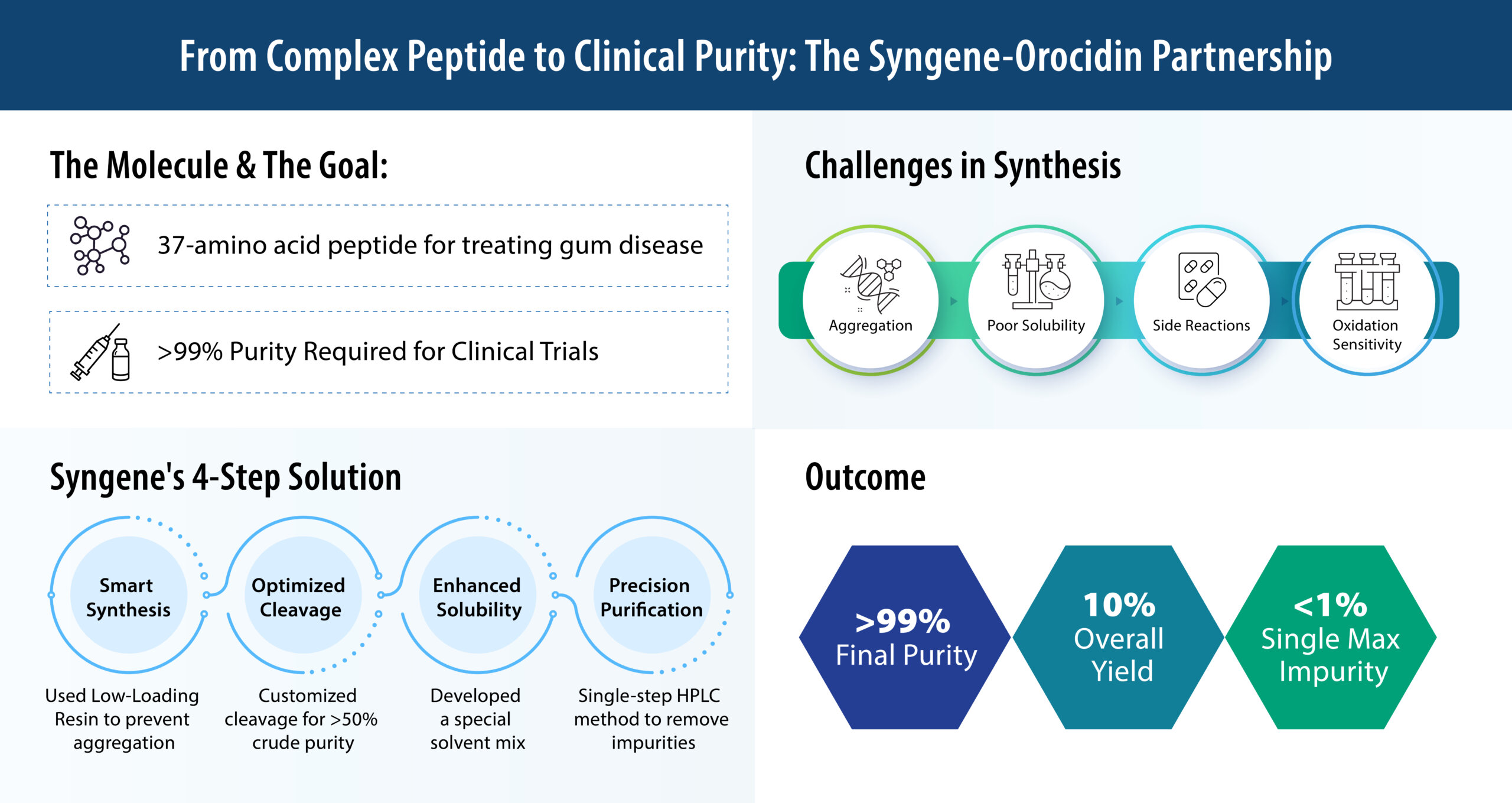
OROCIDIN A/S, a company focused on developing a novel treatment for advanced gum disease, or aggressive periodontitis, partnered with Syngene for the synthesis of their lead molecule, QR-01. QR-01 is a 37-amino acid (37-mer), cationic, amphipathic peptide with a unique structure that allows it to effectively target and eliminate harmful bacteria. To bring QR-01 into clinical trials, Orocidin needed to produce a high-purity peptide suitable for human use, with a target purity of greater than 99% and a single maximum impurity of less than 1%.
The Challenge: Overcoming Synthesis Obstacles
The development of this 37-mer peptide presented several significant challenges due to its unique chemical properties. The peptide is rich in hydrophobic and basic amino acids, which made it prone to aggregation during Solid-Phase Peptide Synthesis (SPPS). This aggregation can lead to incomplete coupling and a low crude yield. Additionally, the N-terminal region of the peptide contains multiple lysine and arginine residues, which made it susceptible to incomplete deprotection and other side reactions. The crude peptide was also highly sensitive to oxidation and poorly soluble, forming aggregates in aqueous solutions and making purification difficult.
Syngene’s Solution: Tailored Synthesis and Purification
To overcome these challenges and achieve the high-purity standards required for clinical trials, Syngene’s chemical development team created a customized synthesis and purification process.
Used Low-Loading Resin During Synthesis
To prevent sequence-induced aggregation during SPPS, Syngene used a low-loading resin. This strategy reduced the concentration of the growing peptide chains on the resin, thereby minimizing the aggregation-prone interactions and improving the overall efficiency of the synthesis.
Customized the Chemical Cleavage Step
Syngene optimized the cleavage cocktail to prevent the formation of TFA adducts and other side reactions, which are common with arginine-rich peptides. This optimization resulted in a crude peptide with a purity greater than 50%.
Improved Solubility for Smoother Purification
The crude peptide was difficult to dissolve in water. Syngene developed a specialized solvent mix to improve its solubility and enable easier purification. This made it possible to prepare the peptide for the next crucial purification step.
Developed a Tailored, Single-Step Purification Method
To achieve the required purity, Syngene developed a customized, single-step reverse-phase HPLC purification. This method used a tailored gradient to efficiently resolve co-eluting impurities, allowing for high-purity results without compromising the yield. Using this single-step approach, Syngene was able to achieve a final peptide purity of over 99%, with a single maximum impurity of just 1% and total impurities of 0.6%.
From Lab-Scale to GMP Manufacturing
After successfully achieving the purity requirements at a small scale, Syngene seamlessly transitioned to cGMP manufacturing at Orocidin’s request. The SPPS-based synthesis and single-step HPLC purification process were scaled up while maintaining both quality and consistency. Despite the inherent complexity of the 37-amino acid peptide, Syngene achieved an impressive overall yield of 10%. Stability staging was also initiated to ensure long-term compliance. This effort showcases Syngene’s capability to deliver clinically compliant peptides with high reproducibility, setting a new benchmark for cGMP-grade antimicrobial peptide production.