(Based on the research article “In Silico Phototoxicity Prediction of Drugs and Chemicals by using Derek Nexus and QSAR Toolbox” by Ahuja et al.).
Challenge of Phototoxicity
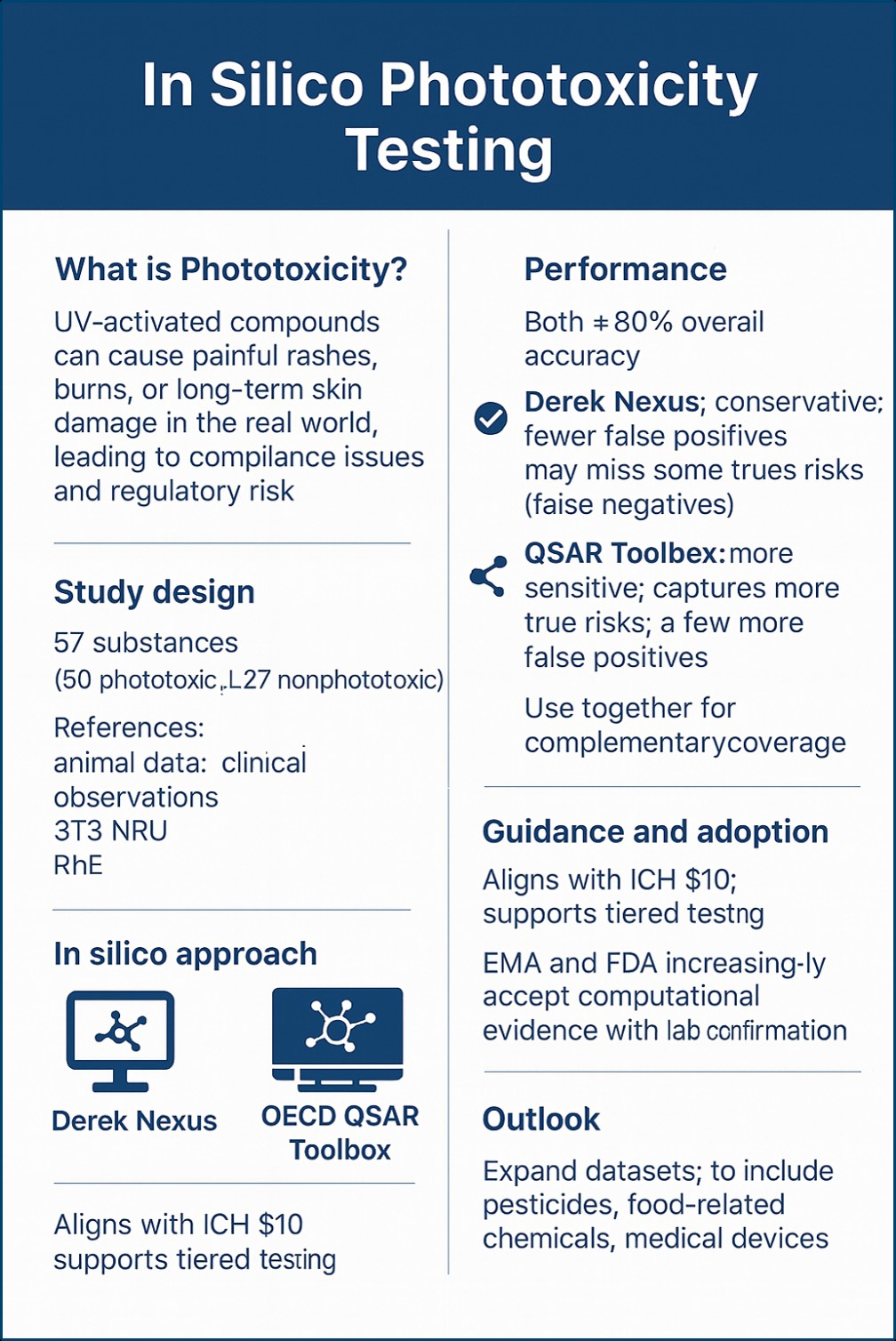
One of the less obvious risks in drug development is not how a medicine works inside the body, but how it behaves in the real world, particularly when exposed to sunlight. Certain compounds, on activation by ultraviolet (UV) radiation, trigger painful rashes, burns, or long-term skin damage. This phenomenon, known as phototoxicity, results in reduced patient compliance, the abandonment of promising drug candidates, and regulatory challenges. In drug development, phototoxicity testing determines how a medicine behaves when exposed to sunlight.
Traditionally, phototoxicity testing has relied on animal models (mice, rats, guinea pigs) or established in vitro assays. While effectve, these methods are slow, costly, and ethically challenging. Each compound tested requires multiple experiments, skilled staff, animal housing facilities, chemical supplies, and specialized laboratory infrastructure. For industries where every month of delay can mean millions in losses, these traditional methods are becoming less sustainable. Moreover, with growing global pressure to minimize animal testing, there is an urgent demand for faster, cost-efficient, and ethically acceptable alternatives.
In Silico Testing Development with Derek Nexus and QSAR Toolbox
This is where in silico testing comes in. “In silico” simply means “within the computer.” Instead of running lengthy experiments in a lab, scientists feed the chemical structure of a compound into advanced predictive software and obtain a forecast of whether that compound is likely to cause phototoxic reactions. In this study, the team chose two recognized systems: Derek Nexus and the OECD QSAR Toolbox.
The researchers collected a diverse panel of 57 substances, including 30 known to cause phototoxicity and 27 known to be nonphototoxic. Notably, the researchers also had reference data for these compounds, including results from earlier animal studies, human clinical observations, and laboratory assays such as the 3T3 phototoxicity assay (Neutral Red Uptake test) and the reconstructed human epidermis (RhE) model, which mimics human skin. The goal was clear: evaluate if in silico platforms can speed up phototoxicity screening, delivering rapid, economical assessments that complement or partially replace traditional laboratory testing.
Results: Phototoxicity Testing Promises and Limitations
The results reveal that, for phototoxicity testing, Derek Nexus tends to be more conservative, rarely raising false alarms, but sometimes missing true dangers. QSAR Toolbox is more sensitive, catching more true risks, though at the cost of a few more false alarms. Therefore, used together, they provide a complementary safety net.
The findings of the study highlight the maturity and promise of computational toxicology. Both Derek Nexus and the QSAR Toolbox reached overall predictive accuracies of nearly 80%, which is impressive given the complexity of phototoxic mechanisms. These results suggest that in silico tools can be meaningfully integrated into early-stage safety evaluations. By identifying compounds with phototoxic potential before advancing to costly in vitro or in vivo testing, researchers can conserve resources, reduce animal use, and accelerate decision-making in drug development pipelines.
The study also aligns with the ICH S10 guideline on photosafety, which encourages early assessment of UV-related risks. Notably, regulators such as the EMA and FDA are increasingly open to computational methods as part of a tiered testing strategy, especially when combined with laboratory confirmation.
Despite these promising results, the study acknowledges some important limitations. In silico models work only when the chemical structure of a compound is known. They are less effective at predicting the behavior of complex mixtures, such as botanical extracts, cosmetic blends, or essential oils, where multiple components interact. Moreover, while 80% accuracy is valuable, the potential consequences of a false negative (a genuinely harmful drug passing as safe) mean that these tools cannot fully replace laboratory validation. Instead, they should be viewed as a powerful first filter, guiding where to focus experimental resources.
Further validation across a broader range of compounds, including medical devices, pesticides, and food-related chemicals, will help strengthen confidence in these models. As databases expand and algorithms improve, predictive accuracy can be expected to increase.
Evaluating the Significance of This Study
This study signals a crucial shift in modern toxicology. By combining computational prediction with established laboratory and clinical data, the researchers demonstrated that the future of safety testing lies in integration, not replacement. In silico tools like Derek Nexus and the QSAR Toolbox offer rapid, cost-effective, and ethically favorable screening. They do not eliminate the need for laboratory work, but they make the process smarter, faster, and more efficient.
For the pharmaceutical, cosmetic, and chemical industries, the benefits are profound. As phototoxicity testing becomes increasingly streamlined, development costs can be reduced, regulatory compliance can be improved, and, most importantly, patient safety can be better safeguarded. When medicines meet sunlight, smarter phototoxicity testing—from in silico testing to the 3T3 phototoxicity assay—keeps the story about health and healing, not hidden harm.
Reference
Ahuja V, Adiga Perdur G, Aj Z, Krishnappa M, Kandarova H. In Silico Phototoxicity Prediction of Drugs and Chemicals by using Derek Nexus and QSAR Toolbox. Alternatives to Laboratory Animals. 2024;52(4):195-204. doi:10.1177/02611929241256040