Summary
Pharmaceutical companies are under immense pressure to develop drugs for unmet medical needs while improving processes to shortentimelines and minimize costs.
This case study explains how Syngene minimized costs while ensuring quick turn-around time when scaling a client’s compound from lab-scaleto cGMP- scale.
Syngene not only delivered as per timelines but also increased yield by 25% and reduced the overall drug product cost by 65%.
About the client
The client is a leading global pharma company that uses innovative science and digital technologies to create transformative therapies.
Its products reach nearly one billion people worldwide. The company is also constantly working to discover new ways to expand treatment access to those who need it the most.
The challenge
Syngene’s chemical development team received a proposal from the client to develop a quick, scalable, safe, and cost-effective process for taking their compound from lab-scale to cGMP-scale.
The compound, developed by the client’s offshore Medicinal Chemistry team, was based on seven linear synthetic steps with a low average yield of around 7%. The process for drug development also comprised five chromatographic purification steps as a result of using toxic and hazardous chemicals during route development.
Additionally, the key starting material (KSM) was quite expensive and likely to jack up the cost of the final drug product.
The client was looking for a suitable route modification process that could demonstrate the scalability and safety of the final drug product at minimum cost.
The project objectives and milestones were as follows:
- Develop a quick, robust, and scalable process suitable for large-scale synthesis
- Deliver 80 to 100 grams as proof of concept (PoC) along with a technology package
- Deliver 1 kg of the drug product under cGMP environment
- Drug development in five to six weeks, followed by PoC and kilo-scale delivery for Tox studies
Syngene’s holistic approach to process development
A team of highly skilled process chemists, analysts, process engineers, process safety engineers, quality, production, and project management came together to decide on a holistic approach to process development. This included the following:
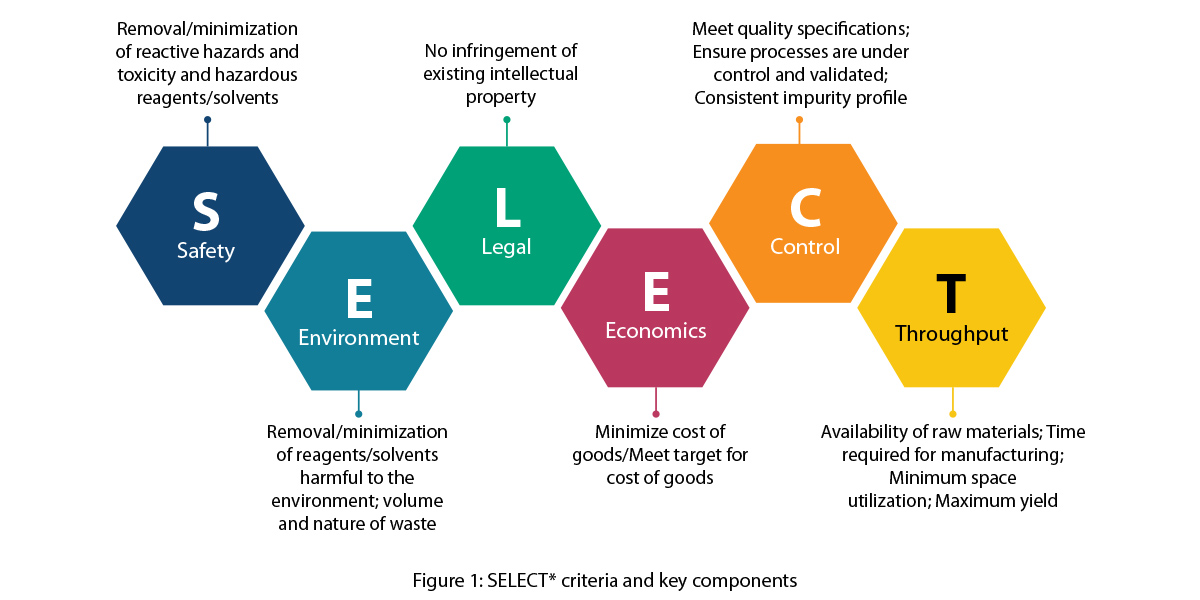
- Review of the current process in detail and listing out the challenges in terms of safety, yield, impurity profiles, reproducibility, scalability, environment, and process economics using the ‘SELECT’ criteria
- An exhaustive literature survey to support process understanding
- Interpretation of the process in terms of challenges, opportunities, and probable solutions
- Detailed experimental plan with alternate routes based on literature and brainstorming with the team
- Detailed plan split into five sections – process development; process optimization; analytical method development and validation; safety
assessment; scale-up and progress monitoring plan - Activities planned in a series and in parallel, based on the advantages and complexities involved
SELECT criteria – An ideal process condition approach
Syngene decided to adopt the industry standard ‘SELECT’ criteria to ensure a 360- degree approach to process development. This criteria is
extensively used at Syngene across client projects to make sure no parameter is missed out during development and scale-up. The Syngene
team judiciously followed the criteria and came up with a process plan as shown below.
*Butters, M., Catterick, D., Craig, A., Curzons, A., Dale, D., Gillmore, A., & White, W. (2006). Critical assessment of pharmaceutical processes a rationale for changing the synthetic route. Chemical Reviews, 106(7), 3002-3027.
The process would ideally have the following characteristics:
- Minimal number of synthetic steps- preferably convergent routes
- Bare minimum cryogenic operating conditions
- No requirement for special equipment
- High conversion, selectivity, and yield at every step
- Easy isolation methods and robust crystallization steps
- Avoid chromatographic steps wherever possible
- Avoid using hazardous reagents and reaction conditions
- Use low cost and readily available raw materials
- Minimize protecting and deprotecting steps, wherever possible
The Solution: Mapping process to project outcomes
Criteria | Existing Process | The Opportunity | The Solution | Project Outcome |
|---|---|---|---|---|
Economics and throughput | Linear synthesis | High risk associated with each step and need for quick turn-around time | To make the route more
convergent to minimize process
risk | Developed a convergent route
with minimal tweaking and
reduced risk towards delivery |
Economics | Overall yield of around 7 % | • Uneconomical and low yield • Need for atom economy | Process optimization via DoE
(Design of experimentation)
methodology | • Optimized the process via DoE • Established process parameters having statistical significance • Demonstrated higher yield and selectivity (> 25%) |
Safety, economics, and environment | High solvent volumes and
class- 1 solvent employed in one
of the process steps | • High solvent volumes
posing economic
challenges • Class 1 solvent posing high toxicity levels and deleterious environmental effect | • Bring down the volumes based
on process optimization • Eliminate Class 1 solvent with appropriate Class 3 solvent through solvent screening study | • Solvent volumes reduced by
1/3, Reagents by 2/3 • Class-1 solvent replaced appropriately with Class-3 solvent |
Throughput, environment, and economics | Chromatographic purification steps | Highly unscalable
process, especially for
commercial supplies | • Explore the in-situ process
wherever possible and minimize
the need for purification till the
end. • Develop crystallization process wherever possible | • Successfully eliminated 4 out of
5 chromatographic steps
• Developed in-situ process
through a crystallization
process
• Achieved purity requirement
of around 95-98% for the final
drug product |
Safety and
environment | Use of toxic
and hazardous
compounds | Safety risk from handling
the drug product on a
large scale | • Assess the risk through
systematic safety studies such
as desktop screening and
quantitative methods • Develop a comprehensive risk assessment program | • Conducted detailed
quantitative risk assessment
using DSC, RC1e, vent sizing,
and so on to arrive at a holistic
process condition
• Demonstrated the process at
scale while ensuring minimal
process risk in terms of timely
and safe delivery of the
compound |
Economics | High cost of key
starting material
(KSM) | High impact on process
economics | • Explore alternative KSMs which
are readily available and low cost. • Perform a cost-benefit analysis | • Identified an alternative KSM
for the process which could
perform with equal efficiency. • Reduced costs by 65% • Identified a reliable supplier for uninterrupted supply of the KSM |
Throughput
and control | Absence of
reliable analytical
methods;
Difficult to
reproduce | Reproducibility of the
analysis | • Develop robust analytical
methods using HPLC, GC
wherever applicable | • Developed robust and
analytical methods for
in-process monitoring and final
analysis |
Conclusion
Syngene not only reduced the cost of the final drug product by almost four times but also scaled up operations from lab-scale to cGMP scalein a short span of time.
The process involved identifying a new low-cost starting material and developing a new synthetic route using fewer steps to deliver the final drug product. The new process resulted in an increased yield of around 25% compared to the earlier yield of only 7%. Syngene was also able to reduce solvent volumes and turn-around time while increasing reproducibility. Additionally, we were able to eliminate toxic reagents from the compound, thereby facilitating a safe process. We also identified a supplier for the starting material to ensure uninterrupted supply for the client.
The entire process was developed in five weeks, in tune with project requirements. Syngene delivered 85 gms of the product during PoC stage and also successfully scaled up operations to kilo-scale in a cGMP environment.