Overview
Syngene collaborated with a clinical-stage biopharmaceutical organization focused on advancing targeted protein degradation therapies. Their mission is to develop next-generation medicines that transform patient outcomes, particularly in oncology. By leveraging a proprietary platform, the company designs and optimizes small-molecule therapeutics aimed at challenging disease targets, including those traditionally considered undruggable.
Scientific Background
Chiral inversion refers to the transformation of one enantiomer into its mirror image, a process that can significantly influence a drug’s pharmacological profile. This enzymatic conversion may alter efficacy, induce toxicity, and affect drug stability, making it a critical consideration in pharmaceutical development.
Study Objectives
The primary goal was to assess the chiral interconversion of a racemic compound (R) and its isomers, E1 and E2, through both in vitro and in vivo studies. In vitro evaluations were conducted using rat, cynomolgus monkey, human plasma, and HiBIT media. The HiBIT assay medium was specifically chosen since compound potency assays were performed in this media. Additionally, an in vivo study was performed in rats to determine the pharmacokinetic profile and extent of isomer interconversion following a 10 mg/kg oral dose of the racemate.
A robust, selective, and reproducible LC-MS/MS-based chiral bioanalytical method was developed to quantify the isomers. Key chromatographic parameters—including stationary phase, mobile phase composition, temperature, and flow rate—were optimized to ensure precise peak separation
Syngene’s Integrated Solution
Syngene provided comprehensive support through its advanced Drug Metabolism and Pharmacokinetics (DMPK) facilities, enabling seamless execution of both in vitro and in vivo studies. This integrated approach ensured timely generation of high-quality data aligned with stringent scientific standards
Study Design: in vitro studies
- Matrices: Rat, monkey, and human plasma; HiBIT assay medium
- Concentration: 500 ng/mL
- Incubation Temperature: 37 °C
- Time Points: 0, <2 min, 10 min*, 30 min*, 1 h, 3 h, 8 h, 24 h
- Analysis: LC-MS/MS using a chiral method
- (*Additional time points for human plasma)
Study Design: in vivo studies
- Animal Model: Male Sprague-Dawley rats
- Formulation: 20% PEG 400, 20% SBECD (Captisol), 60% water (pH 3.5)
- Dose: 10 mg/kg, 5 mL via oral gavage
- Sampling: Jugular vein, serial sampling
- Anticoagulant: Sodium citrate (200 mM, pH 4.79, ~6% v/v)
- Time Points: Pre-dose, 0.25, 0.5, 1, 2, 4, 8, 24 hours
Study Design: in vivo studies
The LC-MS/MS method employed an amylose-based stationary phase with acidified aqueous and organic mobile phases under isocratic conditions (A:B, 20:80 v/v).
Key Findings
- In Vitro studies: Rapid interconversion was observed across all matrices. In rat plasma, a 1:1 ratio was reached within 3 minutes, with a
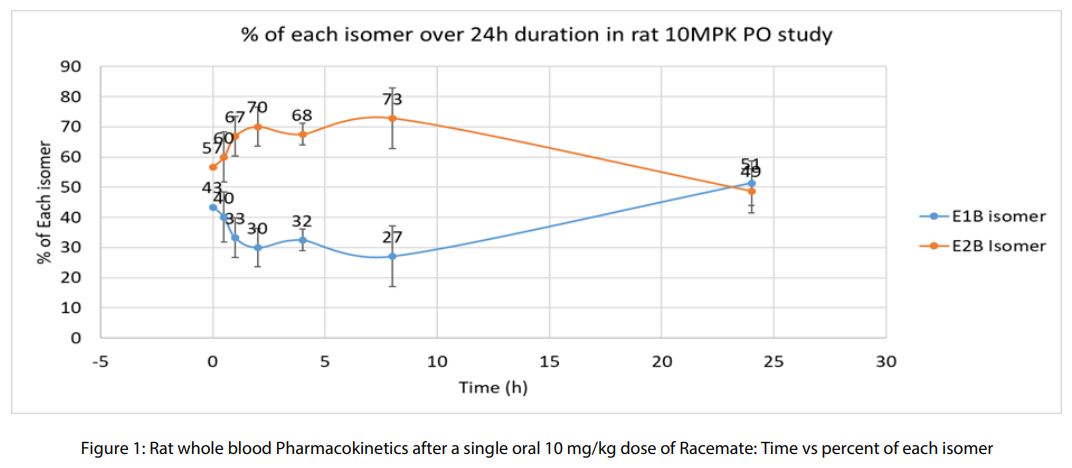
shift toward E2 by 24 hours. Monkey and human plasma showed even stronger bias toward E2 formation. HiBIT medium also demonstrated a modest preference for E2. - In Vivo studies: Following oral administration, E2 predominated during peak sampling hours, with a return to a 1:1 ratio by 24 hours.
- Bioanalytical assay: Chromatographic peak separation was achieved for both isomers (E1 and E2) on a chiral stationary phase. Retention-times were 10.69 min for E1 and 8.57 min for E2.
Conclusion
Syngene’s DMPK team successfully developed a rugged chiral bioanalytical method with adequate resolution and sensitivity, ensuring accurate quantification of isomers. The experimental teams executed both in vitro and in vivo phases with precision, yielding robust and reproducible data.
The studies revealed species-specific differences in isomer stability and interconversion, with a consistent trend favouring E2 formation. These insights are critical for understanding the pharmacological behaviour of the compound and guiding future development strategies.