About the client
The client is a biopharmaceutical company dedicated to developing novel bile acid modulators to treat orphan paediatric liver diseases and other liver and gastrointestinal disorders. They focus on discovering and optimizing compounds with potential therapeutic impact.
The challenge
The client had advanced two compounds for further development into the clinical stage — Compound A, an already marketed bile acid modulator, and Compound B, an investigational optimized lead molecule. The client aimed to identify the superior candidate in terms of pharmacokinetics (PK) and pharmacodynamics (PD). To this end, a critical preclinical evaluation was necessary. For this, the client wanted to perform a 7-day repeat-dose pharmacokinetic (PK) study in mice for the two compounds.
The study had two major challenges:
- The compounds had to be administered through food pellets, as the client had observed signicantly less stress response fromconsuming pellets than from conventional injection methods (e.g., subcutaneous, intraperitoneal, or intravenous). However, thisapproach necessitated stringent monitoring of pellet consumption, timing, and consumption amounts.
- Additionally, the data had to be obtained from multiple study domains, such as in vivo studies, bioanalytical analysis, and in vivo pharmacology, that needed close collaboration among multiple teams to get a consolidated view of the data. This is a major challenge for many contract research organizations (CROs), who typically struggle to coordinate such multifaceted studies.
The solution
Syngene offered a robust solution by providing a collaborative platform that facilitated data integration across multiple domains. The study design included three experimental groups, each consisting of five mice, as outlined below:
| Group | Treatment | No. of mice |
| G1 | G1 Chow Diet (Control) | 5 |
| G2 | Chow Diet with 0.004% of Compound-A (Cpd-A) | 5 |
| G3 | Chow Diet with 0.013% of Compound-B (Cpd-B) | 5 |
Three independent teams within Syngene’s Discovery Biology division worked cohesively to execute the study. The DMPK in-vivo team executed the in-life phase by administering the diet, collecting samples at predetermined times, and computing the PK parameters. The bioanalytical team quantied the test compound exposure in plasma and assessed serum C4 levels (a bile acid precursor). The in-vivo pharmacology team evaluated total bile acid concentrations and conducted gene expression analyses for CYP7A1 (a liver enzyme involved in bile acid biosynthesis) and FGF-15 (a regulator of bile acid synthesis in the ileum).
Observations
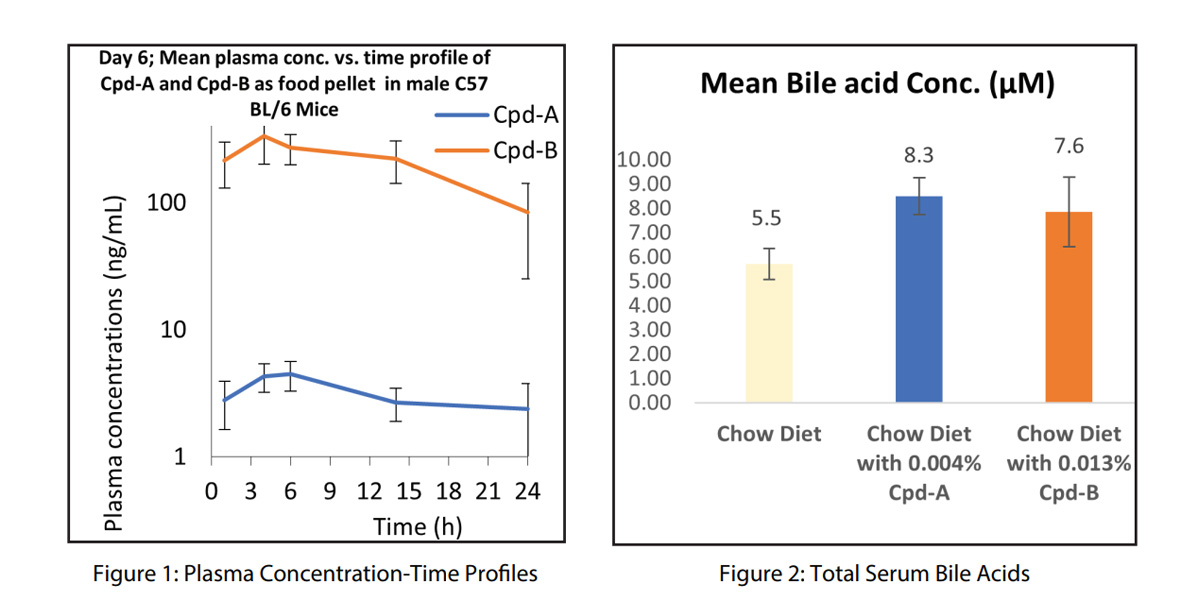
The pharmacokinetic analysis (Table 1. Fig 1) revealed that Compound-A exhibited a mean AUC_last of 70 hng/mL with a Cmax of 4.8 ng/mL as expected, based on previous data. In contrast, Compound-B demonstrated significantly higher plasma exposure, with a mean AUC_last of 4885 hng/mL and a Cmax of 350 ng/mL. These results confirmed that Compound-B had a much greater systemic exposure than Compound-A, suggesting it might offer superior efficacy.
Compound ID | Mean Cmax (ng/mL) | Mean AUClast (h*ng/mL)` | Serum C4 Concentration |
|---|---|---|---|
Chow Diet | — | — | 9.3 ng/mL |
Cpd-A | 4.8 | 70 | 10.96 ng/mL |
Cpd-B | 343 | 4885 | 12.36 ng/mL |
Table 1: PK Data and Serum Biomarker Concentrations
Compound B showed a multifold higher plasma exposure, as shown in Fig.1, with total bile acid levels consistent across all groups (Fig 2). Also, the serum C4 levels on Day 7 remained similar across all groups. This observation suggested that while the pharmacokinetic proles of the compounds were distinct, the impact on serum C4, as a biomarker of bile acid metabolism, was minimal across treatments. This was consistent with our learning that serum biomarker C4 modulations are more noticeable in OATP knock-out mice than in wild-type mice.
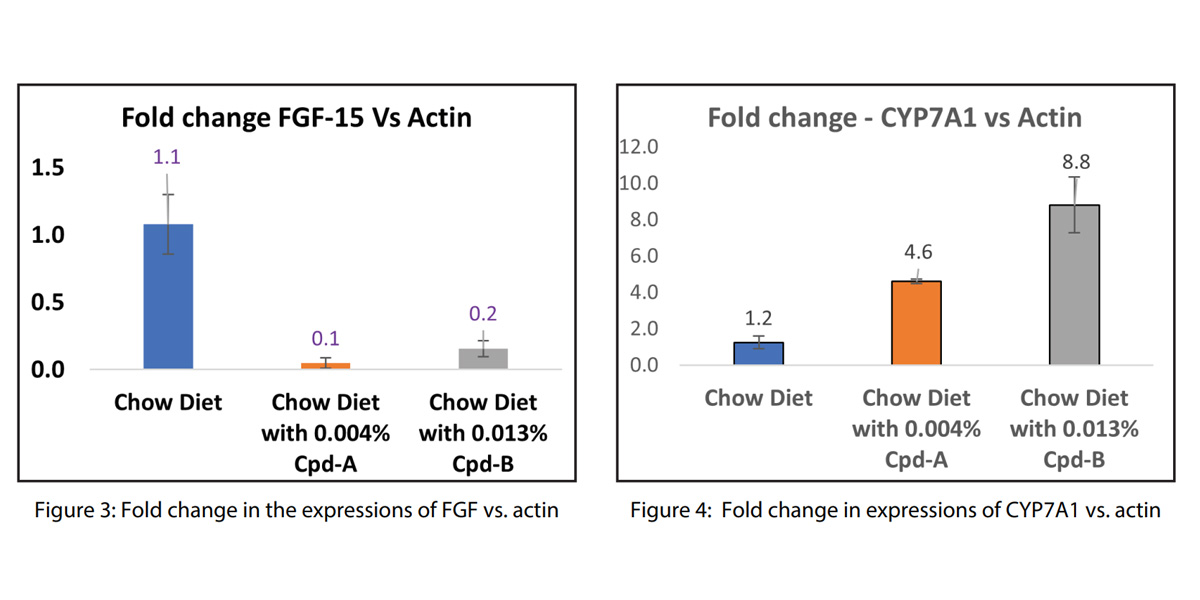
Interestingly, the gene expression analysis showed a notable decrease in ileum FGF-15 expression (Fig. 3) and an increase in liver CYP7A1 expression (Fig. 4) in both Compound-A and Compound-B group compared to the control group, and the change in CYP7A1 was more pronounced in Compound-B group. The fact that both these enzymes are involved in the biosynthesis of bile acid indicates that compounds A and B were involved in bile acid modulation primarily by impacting bile acid synthesis.
Interestingly, the gene expression analysis showed a notable decrease in ileum FGF-15 expression (Fig. 3) and an increase in liver CYP7A1 expression (Fig. 4) in both Compound-A and Compound-B group compared to the control group, and the change in CYP7A1 was more pronounced in Compound-B group. The fact that both these enzymes are involved in the biosynthesis of bile acid indicates that compounds A and B were involved in bile acid modulation primarily by impacting bile acid synthesis.
Conclusion
The comprehensive data generated from this study allowed the client to make informed decisions about advancing compound B into the next stage of development for treating bile acid disorders. The pharmacokinetic proles, serum biomarker data, and gene expression analyses aligned with the client’s expectations that both compounds were involved in bile acid modulation. However, compound B had signicantly higher plasma exposure levels, indicating that it could have a better therapeutic potential due to better plasma exposure.
Syngene’s ability to integrate three distinct domains—PK, PD, and molecular expression—into a cohesive study is a key dierentiator in this project. The project demonstrates our expertise in executing complex preclinical studies, consolidating data across multiple functional teams, and providing comprehensive insights that inform the client’s development decisions. Further, it exemplies our capability to generate multidimensional data that spans pharmacokinetics, biomarker modulation, and gene expression analysis, positioning the company as a leader in providing holistic drug discovery and development support.
Learn more about our DMPK studies or contact our experts.