The context
Monoclonal Antibodies (mAbs) derived from hybridoma cell lines are integral to therapeutic applications and used to prepare reagent kits. Hybridoma is one of the platforms used to produce monoclonal antibodies (mAbs) against specific antigens
The requirement
The client is a global biotech company that develops and manufactures cell culture media and cell separation technologies for use in stem cell, immunology, and cancer research. To launch new reagent kits essential in cell and gene therapy, the client wanted to partner with a CDMO with a proven track record and extensive experience in biologics manufacturing. The CDMO would also have to have a state-of-the-art manufacturing infrastructure and the capability to provide a customizable solution based on the biotech’s requirement.
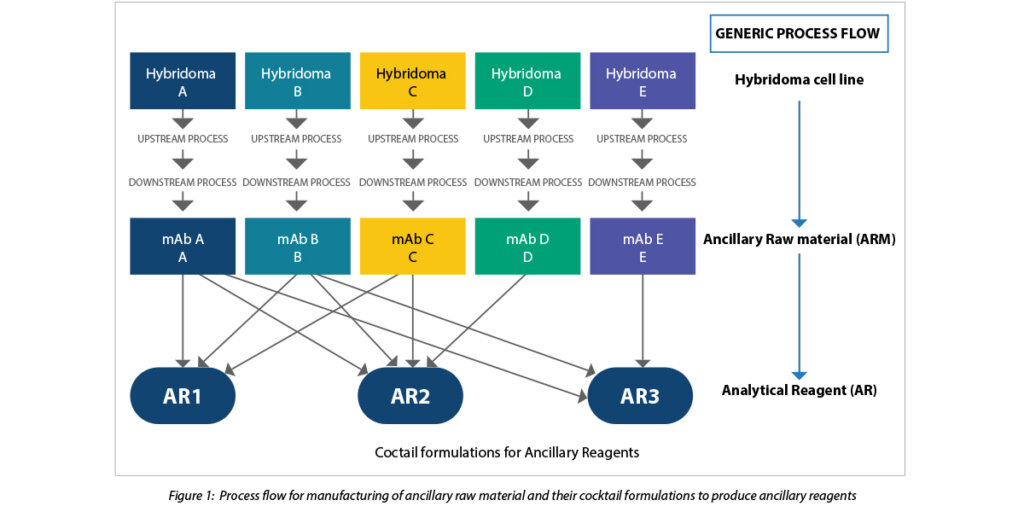
The company approached Syngene to develop this unique manufacturing platform that would ensure the successful integration of hybridoma cell lines with five different ancillary raw materials (ARMs). These ARMs would be used to formulate three ancillary reagents (ARs), which would be a cocktail of these ARMs in defined combinations and at specified concentrations.
The challenge
Hybridoma cell lines pose inherent challenges, such as hybridoma instability, lower antibody yields, heterogeneity in antibody production and scale-up, downstream processing, and regulatory compliance issues.
Addressing these challenges often requires a multidisciplinary approach involving collaboration with scientists from cell biology, process development, manufacturing operations, quality control, and regulatory affairs.
The solution
Our Biologics team curated the technology to produce five ARMs. The ARMs were scaled up to manufacturing scale using single-use platform technology. The ARMs were then used to develop innovative cocktail formulations for creating ancillary reagents (ARs).
The project was delivered right, the first time, resulting in the client meeting the timelines for the launch of the reagent kits. The newly launched reagent kits are expected to play an important role in advancing cell and gene therapy. The kits are designed to activate and expand human T cells for immunotherapy in patients.
Customer benefits
Stable cell line preparation: Development of stable and reliable hybridoma cell lines through successful preparation of master cell banks (MCB) and working cell banks (WCB).
Production process scale-up: Successful scale-up of the production process to meet manufacturing demands, overcoming challenges associated with hybridoma cell lines.
Process consistency and uniformity: Consistency and uniformity in the production of mAbs per customer specifications and global regulatory standards.
Robust platform: Effective platform for producing complex cocktail formulations at manufacturing scale.
Conclusion
This complex hybridoma stem cell project marks a significant achievement in developing mAB cocktail formulations, culminating in the successful production of three ancillary reagents.
The client has integrated these reagents into analytical kits, providing end-users with powerful tools for stem cell identification, characterization, and analysis. The success of this project will enable researchers worldwide to accelerate their stem cell studies, supporting advancements in regenerative medicine, developmental biology, and cellular therapy. As an endorsement of our capability and to strengthen our future collaboration, the client plans to award more such projects to us.
In summary, the project delivered high-quality, market-ready ancillary reagents that are set to make a meaningful impact in stem cell research. Further, our collaboration demonstrates the potential of hybridoma technology in generating critical tools for scientific innovation and commercial application.
To learn more about our mAb manufacturing capabilities, contact our experts.