The challenge
The client is a biopharma company that focuses on early-stage clinical trials. The client had developed a clone that had to be scaled up from lab scale to clinical scale without affecting product quality and titer. The client needed an experienced partner to manage the process complexities, including challenging feed and gassing strategies. Another requirement was to deliver GMP batches of the drug product for use in clinical studies.
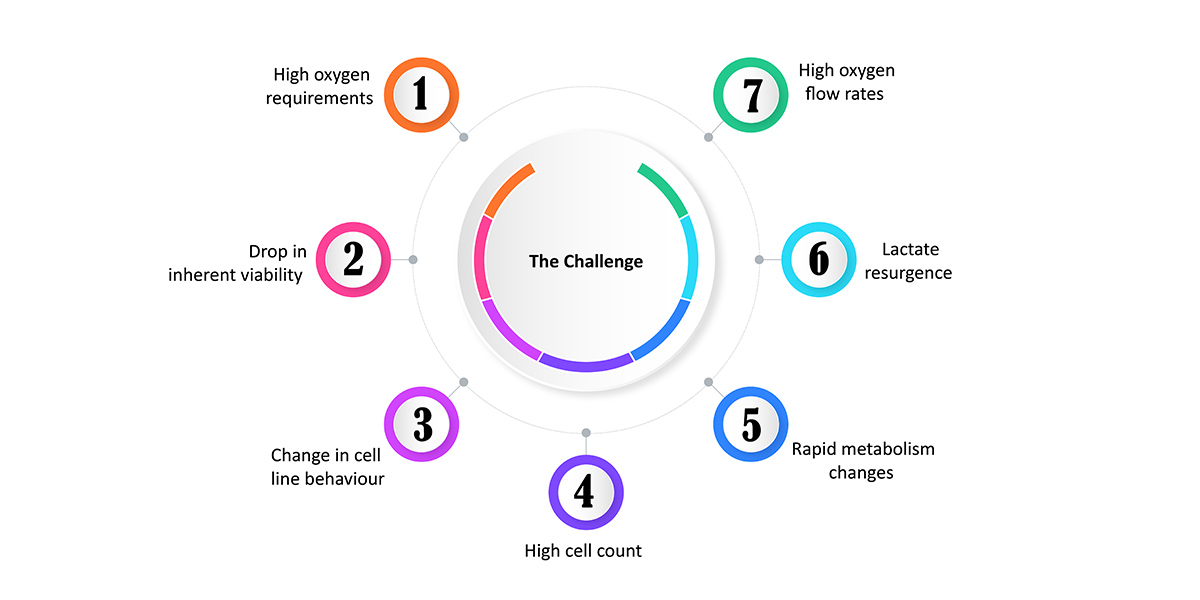
The scale-up process presented the following challenges:
The solution
The client decided to partner with Syngene based on their assessment of our capabilities and our long-standing association as a reliable partner. Our team used a structured approach to arrive at a feasible solution for the client. After multiple client meetings and a review of the technical data provided by the client, we arrived at the problem statement. This was then aligned with the client’s requirement to manufacture clinical supplies of the product without affecting titer and quality.
As a first step, we performed a thorough risk assessment of the manufacturing process to ensure smooth scale-up operations. Based on the identified risks, we optimized the feeding, gassing, and bag design for small-scale manufacturing. Then, using the small-scale data, we scaled the process to a clinical scale with a modified bag design. We delivered batches of the product with minimum challenges in maintaining gas flow or feed. As a result, we could deliver high-quality and high-titer product supplies per client expectations.
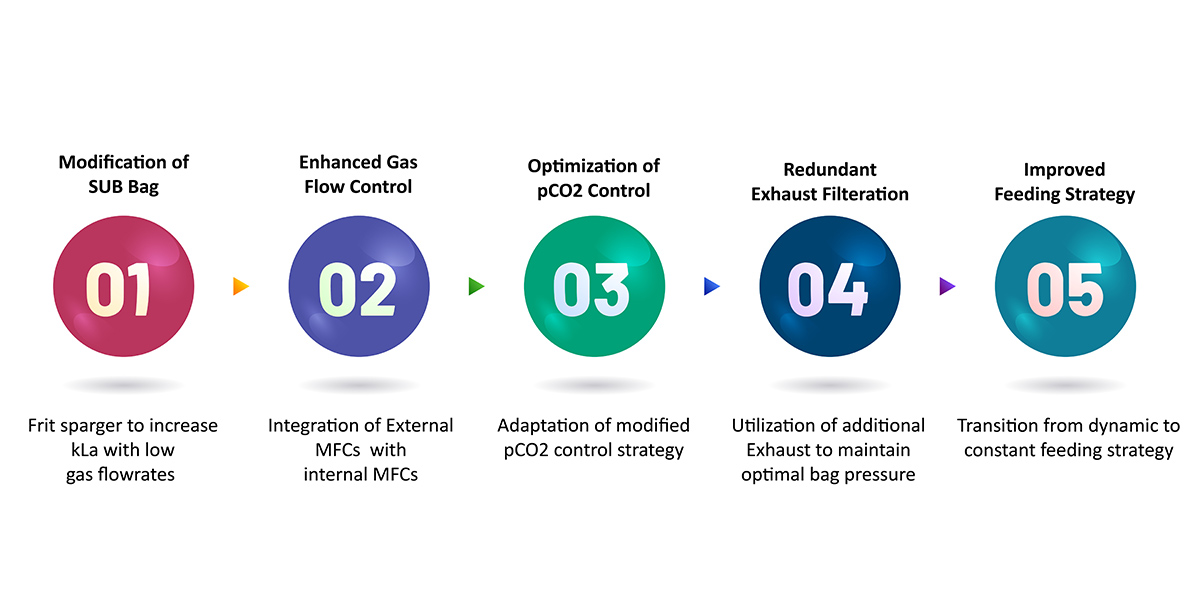
Our solution comprised the following:
Conclusion
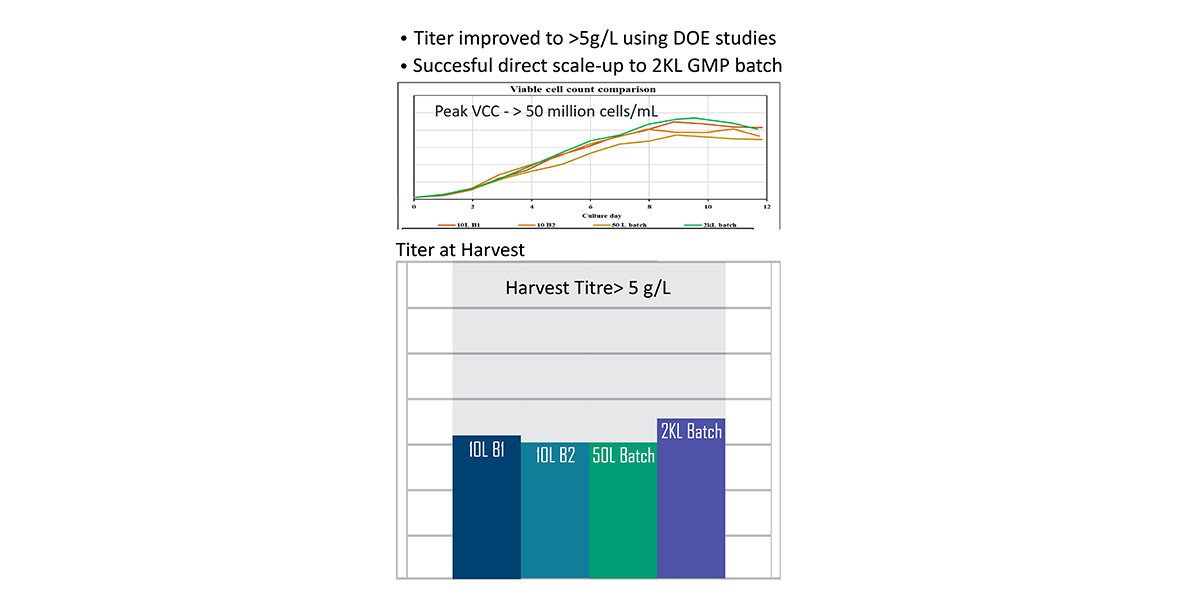
Our solution derisked batch delivery and enabled the manufacturing of the clinical batches of the product with an improved titer of >5g/L.
Further, we achieved successful direct scale-up from clone to 2KL GMP batches of the product.
Titer improvement and direct scale-up to 2KL GMP batches
Explore our biologics development and manufacturing services.
To learn more, contact our experts.