The requirement
A prominent biopharmaceutical company focused on advancing monoclonal antibody (mAb) therapies for cancer treatment was seeking a contract development and manufacturing organization (CDMO) capable of addressing their complex manufacturing requirements. The project demanded expertise in optimizing upstream development processes to generate high-titer clones with stringent quality attributes, all within an aggressive one-year timeline.
Syngene was chosen as the partner for its deep technical expertise in biologics and mAb development. Their decision was further reinforced by our proven track record in delivering high-quality, scalable solutions within tight timelines, supported by well-established platform technologies and methodologies..
The solution
Our team, relying on its prior knowledge and platform approach, acted quickly to deliver a process that would result in a high titer of the desired product quality. The upstream strategy consisted of the following milestones.
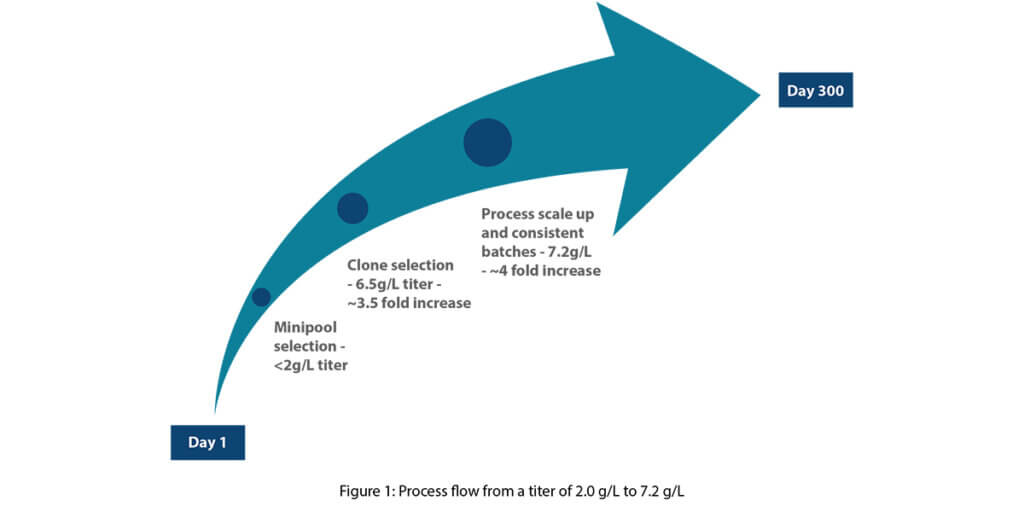
1. Screening and selection of clones
After developing the cell lines, we generated stable pools and evaluated them for titer and potency. This was followed by a selection of monoclones through monoclonal sorting. We then performed monoclonal sorting to select individual clones for further analysis. Subsequently, we performed monoclonal screening to choose the top 10 clones based on product quality and titer.
2. Upstream platform strategy
Our experimental strategy comprised a Design of Experiments (DoE) approach that included two selected clones as one of the six key parameters, alongside medium composition, feed types and percentages, seeding densities, and pH dead bands. A total of 24 bioreactors were utilized for screening, with 22 experimental runs through the DoE as part of a definitive screening design. Additionally, we introduced two conditions based on Syngene’s extensive mAb development experience. After evaluating process attributes such as titer and specific productivity (Qp), we chose ten conditions for a one-step purification process and subsequent product quality analysis. This streamlined approach reduced the number of experiments required to identify high-quality clones capable of industry-leading yields for mAb production.
3. Process scale-up and adaptation
In the final stage of the production process, we scaled up the top condition to 10L bioreactors. We conducted two adaptation batches at this scale, implementing minor process adjustments. During the initial adaptation batch, we achieved a titer of 5800 mg/L. We improved this further to 7000 mg/L by increasing the initial seeding density. Following this, we executed two additional 10L batches, resulting in titers of 7000 mg/L and 7200 mg/L, respectively.
Conclusion
The implementation of our new platform process resulted in a fourfold increase in titer within less than a year, significantly enhancing efficiency and reducing costs for the biopharma company. Our platform approach successfully addressed previous issues related to low yield and lengthy processes, enabling the production of high-quality clones ideal for mAb manufacturing.
The notable increase in titer achieved within such a short timeframe underscores the effectiveness of our methodology. The biopharma company intends to continue collaborating with Syngene to further optimize and scale up its production processes, aiming to achieve clinical success and eventual FDA approval.
To explore how our expertise in mAb manufacturing can benefit your projects, contact our experts.