The challenge
The client is a biopharma company specializing in late-stage process development, technology transfer, scale-up, and manufacturing.
The client had completed clinical trials and was ready to start manufacturing the process performance qualification (PPQ) batches for their biologics when they noticed a difference in product quality as compared to the earlier batches. This was a major setback as they realized that this issue would affect their chances of gaining regulatory approval, affecting product launch timelines.
The client decided to partner with Syngene for a viable solution to achieving uniform product quality.
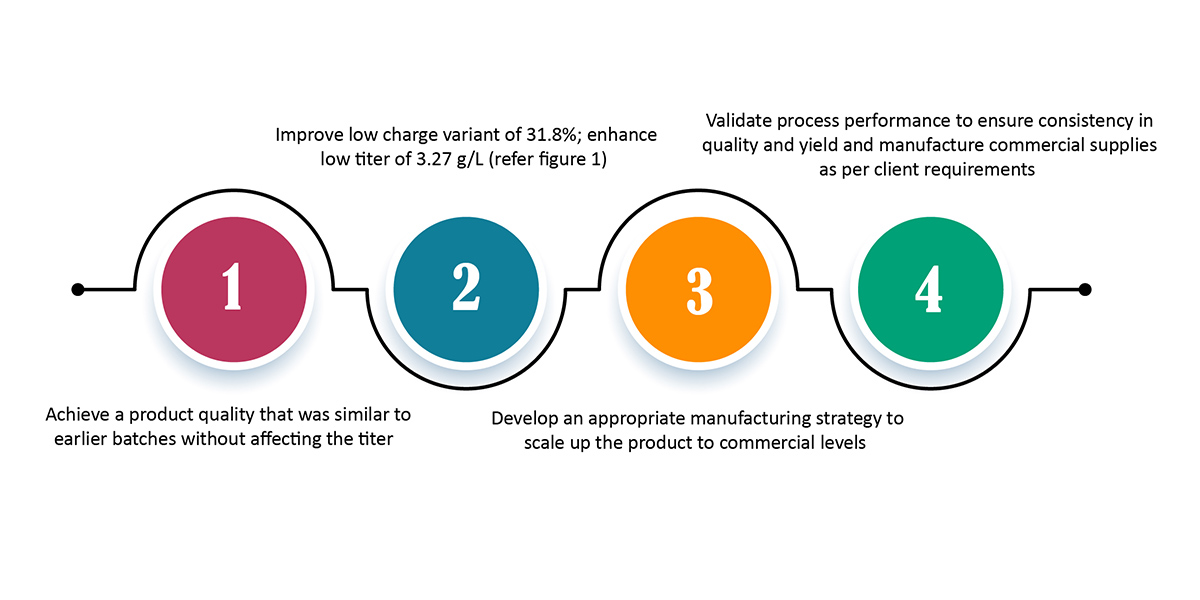
The scope of work was as follows:
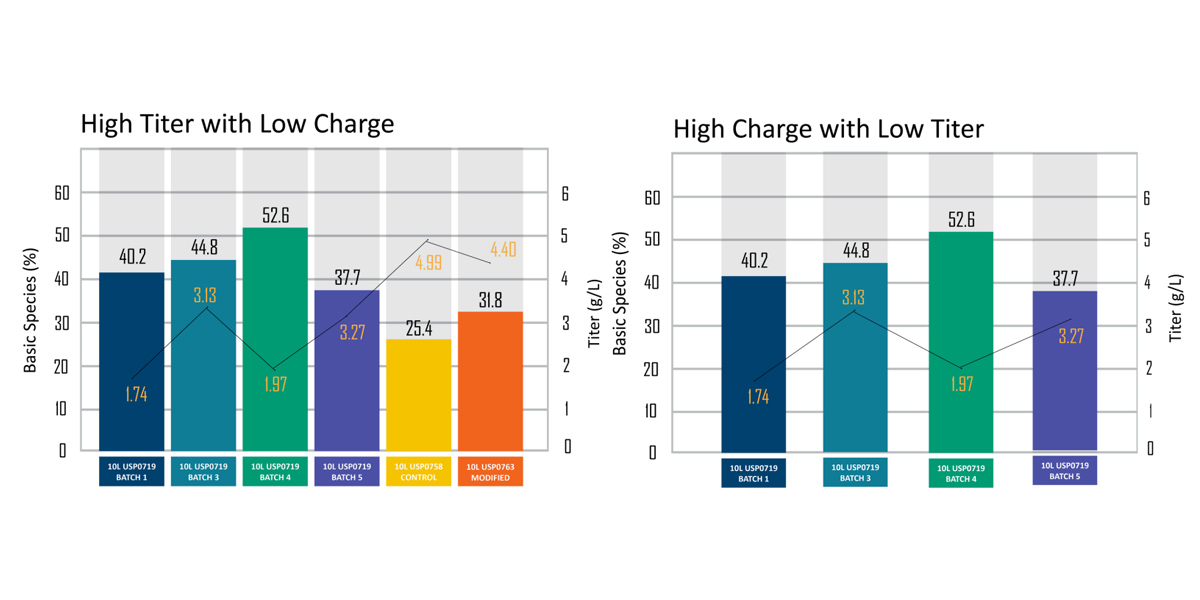
Figure 1: Graph showing product batches of high titer with low charge, and high charge with low titer.
The solution
Syngene used a structured approach to arrive at a viable solution for the client. After multiple client interactions and a thorough review of the technical data, our team arrived at the problem statement. This was then aligned with the client’s requirement of achieving the desired quality levels for regulatory approval.
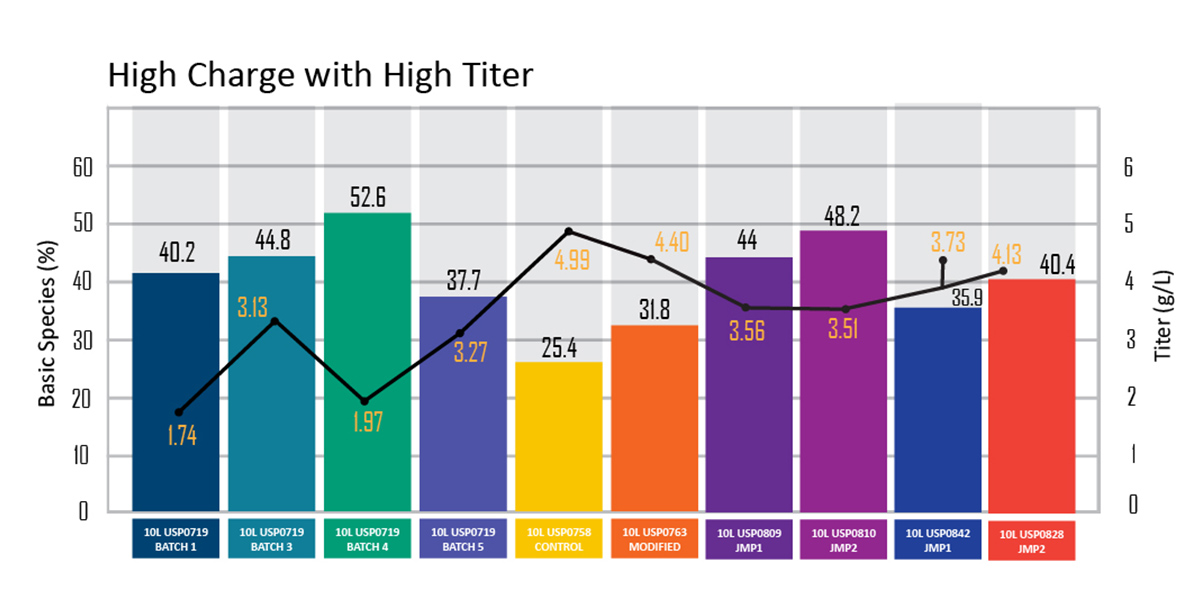
The next step was to develop a scale-down manufacturing model and perform design of experiments (DoE) using different parameters. Then, using the data from small-scale manufacturing, we implemented an appropriate scale-up strategy to scale the manufacturing process to commercial levels. With this methodology, we were able to achieve a consistent process for manufacturing product batches of high charge and high titer — 40.4% and 4.13g/L, respectively.
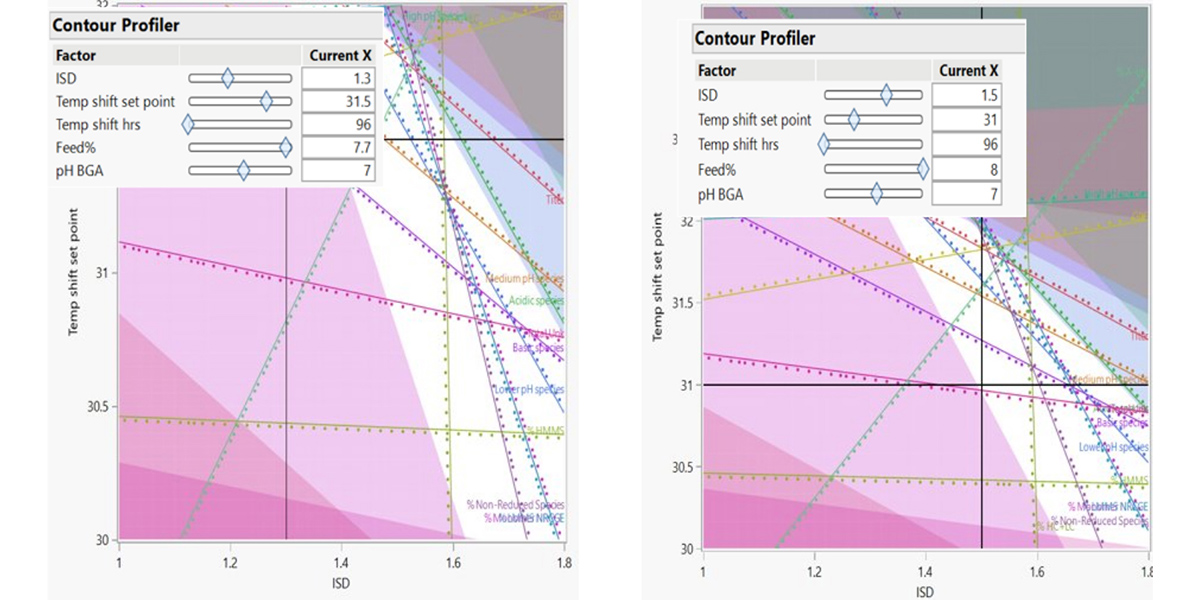
Figure 2: DoE graph showing design space (white space) representing parameter ranges for desired titer and quality attributes.
Figure 3: Graph showing final product batches with significantly improved charge and titer
Conclusion
The client went on to receive regulatory approval for their product from both the FDA and EMA. This enabled timely product launch in the target markets. Patients, in turn, benefited from being able to access essential treatment on time.
This project demonstrates Syngene’s expertise in process scale-up for biologics manufacturing, offering both high-quality and high-titer products. We have a combined capacity of 50,000L SUBs. We also have two high-speed fill-finish facilities capable of manufacturing up to 1 million vials per day with fill volumes ranging from 1 to 100mL.
To learn more about our manufacturing services, contact our experts.