Summary
A global biopharma company partnered with Syngene for Bioanalytical studies for PK, ADA and NAb assays. In less than a year of the collaboration, the company decided to make Syngene, its preferred CRO lab for a novel molecule for migraine. The molecule went on to receive FDA approval in 2018.
Bolstered by the success of this project, the company recommended Syngene to its co-development partner, a global,pharma company. The result was a 3-way partnership between the biopharma company, their co-development partner and Syngene for supporting clinical trials with Pharmacokinetics, Immunogenicity and Neutralizing antibody data using the biopharma company’s proprietary methods.
Today, besides expanding its collaboration with the biopharma company, Syngene is working with their codevelopment partner on 3 novel molecules and 9 clinical studies.
Needless to say, the programs discussed in this case study are excellent examples of how Pharma-CRO partnership can extend beyond company borders into wholistic drug development activities for global licensing.
About the client
The company is a leading American multinational focused on areas of high, unmet medical needs. The company leverages its expertise to strive for solutions that improve health outcomes and dramatically improve people’s lives.
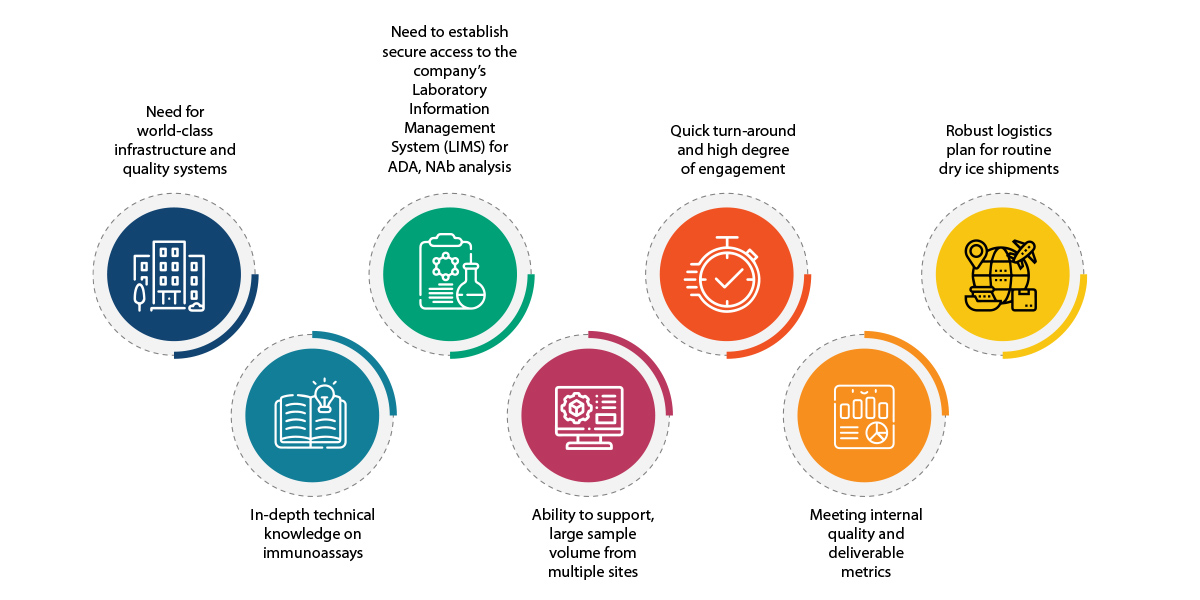
The Challenge
The biopharma company was looking for a reliable partner for outsourcing Bioanalytical (BA) studies for large molecule therapeutics. This was the first time, the company was considering offshoring its GxP Bioanalytical assays to a laboratory outside the US.
As a part of this process, senior decisionmaking staff from the company visited Syngene in June 2012 to review our scientific capability, infrastructure and compliance. Subsequently, a RFP was sent, and Syngene was chosen as their GxP Bioanalytical CRO for Large Molecules.
As a part of technology transfer, a 11-member technical team, was invited to train and transfer ligand binding assays to Syngene. These transfers took place across two locations in USA. The training consisted of familiarization on molecules, certain assay characteristics, critical reagent generation, historical data, best practices and compliance expectations. Assay modalities transferred ranged from ELISA, MSD and cell-based assays to Pharmacokinetics, Immunogenicity and Pharmacodynamics.
At this stage, a collaborative approach was essential to moving the relationship forward. This was more so because, the biopharma company was Syngene’s first international client for its newly formed Large Molecule CRO lab. Yet another challenge before Syngene was aligning with the client’s compliance expectations for 21 CFR part 11 practices.When reviewing Large Molecule laboratories for outsourcing, the company also had the following expectations:
The Solution
Need for world-class infrastructure and quality systems:
- This already existed in Syngene. The client’s GLP compliance team audited Syngene facilities and approved it
In-depth technical knowledge on immunoassays
- Syngene scientists demonstrated capability and skill in Large Molecule regulated bioanalysis over multiple telephonic and in-person interactions
- Our scientists were able to demonstrate troubleshooting aptitude and ability
- Syngene met the stringent cross-validation data expectations of the client in terms of Syngene’s data being comparable with data generated by a North American CRO, the client had partnered with earlier. This was an important milestone for both the companies in terms of building confidence on either side for taking the project forward
Need to establish secure access to the client’s Laboratory Information Management System (LIMS) for ADA, NAb analysis
- Syngene’s IT team worked with the client to establish a Virtual Privat Network (VPN) tunnel to access the company’s LIMS software in a secure way for ADA and NAb analysis
- Using the VPN, Syngene accessed and analyzed over 200,000 samples with high quality data output
- Syngene also achieved 100% success in multiple cross-site validations using automation
Ability to support large sample volume from multiple sites
- We deployed experienced technical project managers and analysts to run and monitor the projects. Constant improvements were made in documentation and processing, as and when required, to minimize errors in documentation and ensure compliance
- To ensure constant reagent supply which was on-time/ahead of project delivery, we collaborated with Indian and global vendors for supply of routine reagents, chemicals and biological matrices based on stringent SLAs.
- Syngene established systems and process to support large volume sample analysis
- Multiple teams were trained to ensure continuity
- Redundancy was ensured by purchase of duplicate instruments and software
Quick turnaround and high degree of engagement
- Syngene met all timeline expectations for QC and QA data for large BLA filing studies
- We supplied high quality data for clinical interpretation and regulatory scrutiny
- Our data successfully passed the client audit test
- All data generated by Syngene further helped the client progress its pipeline to the next milestone
Meeting internal quality and deliverable metrics
- Metrics for supply and quality assurance were collected quarterly, for PK and ADA
- Provided assurance of supply (for a 100% score)
- Method transferred and validated as per agreed timelines
- Ensured troubleshooting time did not exceed or disrupt agreed study timelines
- QC and QA Data provided as per agreed timelines
- Reports provided as per agreed timelines
- Provided quality assurance (for a 100% score)
- Provided number of SOPs, study plans, GLP deviations
- Assay pass rate >80%
- ISR pass rate 100%
- Syngene’s metrics data was compared with other vendors of the client. Our supply and quality metrics was >90%
Robust logistics plan for routine dry ice shipments
The project executed by Syngene is a good example of transition from physical method transfer to a virtual transfer. This was also one of the first BA studies using automation, and full co-location for conducting PK, ADA and NAb assays.
Syngene’s partnership for an FDA-approved molecule
Syngene supported clinical trials for a key molecule of the client with Pharmacokinetic and Immunogenicity data including cell-based neutralizing antibody assays.
The molecule is a medication which targets the calcitonin gene-related peptide receptor (CGRP) for the prevention of migraine. It is a form of monoclonal antibody therapy in which antibodies are used to block the receptors for the protein CGRP, thought to play a major role in starting migraines.
This molecule developed by Syngene, went on to become the first of a group of CGRP receptor antagonists to be FDA-approved in 2018.
The Challenge
For this project, Syngene was tasked with conducting BA studies across PK, ADA and NAb assays. We were required to apply Sandwich ELISA, Bridging MSD and Functional cell-based MSD methods to the project, which were of medium complexity, low to medium complexity, and high complexity, respectively.
The Solution
PK assays
- Syngene identified experienced analysts and project managers, trained to execute a fully validated PK assay.
- Teams were trained on all critical aspects of the Sandwich ELISA method and techniques to ensure best results
- The assay was highly sensitive and the signal to noise ratio was less than 5 times the LLOQ in the Sandwich ELISA method. To ensure consistency in runs with minimal failures, automation was employed for the preparation of standards and quality controls. Assay shifts and changes were also keenly monitored using WATSON LIMS.
Any major assay failure and shift in responses were carefully investigated. Root cause was determined. Corrective and preventive actions were implemented for all future analysis. - PK profile was examined routinely, any outliers (eg. Pre-dose sample showing drug concentration etc.) were immediately called out for clarification
Immunogenicity assays
- ADA assays were conducted with high precision and consistency.
• Thorough validations of the ADA assays ensured suitability and fit for intended purpose - Immunogenicity assay was run in three tiers. Tier one and two comprised binding assays in a bridging format on the MSD platform. Tier three was a functional cell-based assay using ready to plate cells. The method comprised a bioassay step combined with an end-point read out. The read out of the third tier was on the MSD.
- For this program, the client’s LIMS was deployed for the first time via a VPN tunnel for real-time data sharing
Process Improvements
Syngene made several process improvements based on the client’s requirements and regulatory expectations. These included simplification of
assay processes, inclusion of automation, building sample manifest translation software, retrospective validations of software, SOP improvements, streamlining QC oversight, etc.
Throughout the duration of the project, we ensured transparent, clear and effective communication between Syngene and our client. Meetings and bi-weekly calls were scheduled to review progress against various Key Performance Indices(KPIs) and incorporate feedback.
Syngene set up a Governance Body comprising teams of representatives from all aspects of collaboration –– functional , operational and executive teams. The Governance Body reviewed the progress of the collaboration and recommended future strategies for expansion of the collaboration. This ensured alignment with the client’s expectations and requirements at all times.
Syngene’s indigenously developed software helps global biopharma company meet Clinical milestones
An important and much appreciated effort in this collaboration, was Syngene’s indigenously developed software, Manifest re-formatter. Our in-house CSV team along with the IT team, developed this software to reformat Manifest in such a way as to ensure it aligned with Watson-importable format.
Sample e-Manifest is the document which comes in various files formats (i.e. .txt, .xls, .xlsx, .csv) from trial sites. This document contains all the desired information of samples for a study. Re- Formatter is an automated file parser utility which converts the Sample e- Manifest files into to Watson readable file format. The output files are used as input to study import protocol in Watson LIMS only. The software was validated for its intended use to meet the 21 CFR part 11 compliance, and the audit trail enabled, to capture all the events.
As a result of this software, the client’s dependency on clinical CROs to update Manifest has been minimized. This in turn is helping the client meet clinical milestones with ease.
Global biopharma recommends Syngene to its co-development partner, a global pharma company
Encouraged by the success of the first co-developed molecule program, the client recommended Syngene to its co-development partner, a global pharma company, to make Syngene its preferred Large Molecule Bioanalytical Laboratory.
A three-way collaboration was established between the co-development partner, our client and Syngene. The client gave its consent to use
its proprietary PK, ADA and NAb methods for co-development of molecules. The client also provided consent to access its validation reports on PK, ADA and NAb. Data generated based on co-development collaboration was submitted to regulators. The molecule went on to be approved and licensed in the EU.
Syngene’s co-development opportunities with the global pharma company has now further expanded to other areas. Syngene is currently working with the company on 3 novel molecules and 9 clinical studies.
Conclusion
Syngene’s collaboration with the global biopharma company is an excellent example of how Pharma-CRO partnership can extend beyond company borders into wholistic drug development activities for global licensing.
What started as a partnership for outsourced Bioanalytical studies for Large Molecule therapeutics, has now evolved into a co-development partnership, nearing a decade.
In less than a year of the collaboration, we were able to win the client’s confidence into making Syngene the preferred CRO lab for its novel molecule for migraine. This is a testament of our capability –– both in terms of state-of-the art infrastructure and world class scientists –– to deliver solutions at par with global standards.
Our deepening collaboration with our client’s co-development partner, a global pharma company, is also indicative of our growing credibility as a CRO of choice for services across the Drug Development continuum.