The Challenge
A biotech company had developed a fluorescence polarization assay. The company approached Syngene to transfer the assay in an HTS-ready manner and screen a library of 200,000 compounds against a single protein target. Syngene was required to deliver the relevant compounds against a very tight deadline while ensuring high-quality output.
The Solution
Syngene has different instruments to enable HTS solutions. We have multiple HTS-ready plate readers of different makes (PerkinElmer® EnVision®, Tecan Spark®), which can read in autonomous mode once the plates are stacked on the instruments. Further, we have multiple liquid handlers and dispensers (Tecan Freedom Evo®, Beckman Coulter Echo, Thermo Multidrop Combi, Integra Assist Plus), which can be programmed to dispense volumes ranging from nano to micro-liters.
During the HTS assay transfer, the Syngene team encountered a plate uniformity problem. This was due to the inappropriate hardware configuration of the plate reader for the unique fluorophore we were using. Our team worked closely with the manufacturer’s engineering team to make the necessary changes and reconfigure the hardware. As a result, we resolved the issue within a week.
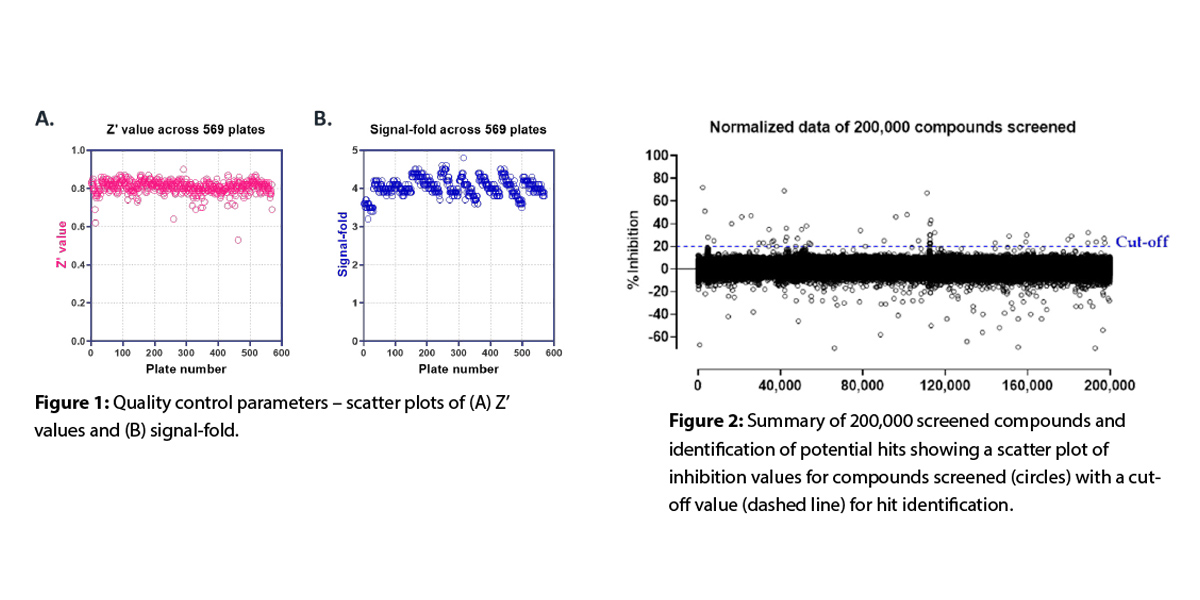
We successfully demonstrated HTS assay transfer by meeting the pre-set assay transfer criteria. As part of the screening, a total of 570 384-well plates were screened within nine days (60 to 90 plates/day). Platewise quality control (QC) parameters, Z’ values are shown in Figure 1. Consistent Z’ values (0.8±0.03) and signal-fold (0.8±0.03) were observed throughout the screen. A summary plot with the percentage inhibition values for all the 200,000 compounds is shown in following Figure 2.
The table below provides a summary of the screenings focusing on critical parameters.
Description | High Throughput Screening |
|---|---|
Library size | 200,000 |
Targets | 1 |
Counter Screen | None |
Total number of 384-plates screened | 569 |
Type of HTS assay | Fluorescence Polarization (Biophysical) |
HTS assay development | client |
Automation and plate uniformity | In-house |
HTS readiness demonstration | Assay transfer |
Number of 384-well plates screened/day | 60 to 90 |
Number of compounds screened/day | 21,120 to 31,680 |
Total number of days of plate screening | 9 |
Average signal-fold observed | 4 ± 0.2 (569 plates) |
Average Z’ for the entire screen | 0.8 ± 0.03 (569 plates) |
Results Delivered
Syngene leveraged in-house expertise and automation capabilities to deliver high throughput screenings of high quality within the agreedupon timelines and at optimum costs. The client was well satisfied with the quality of our output which enabled them to identify hits and proceed to hit triage at an accelerated pace. Commending Syngene on the quality of the work delivered, the client said it was at par with similar work executed by large pharma companies. Further, even after completing this project, the client continued to work with us for routine screening of compounds as part of the hit-to-lead phase.
To learn more about our drug discovery services, contact our team.