The challenge
The client is a large pharma company working on therapies for cardiovascular, metabolism, retinal diseases, immunology, infectious diseases, oncology, and more. The pharma company approached Syngene to generate gene knockouts in human induced pluripotent stem cells (iPSCs) to accelerate their drug research in allogeneic cell and gene therapy.
Human iPSCs are difficult to transfect and are less amenable to efficient gene editing. The challenge before Syngene was to generate gene knockouts of iPSC lines with a high efficiency of >90% using two CRISPR Cas enzymes (Cas9, Cas12a) in multiple iPSC lines.
Some other challenges were as follows:
- Optimizing iPSC transfection protocols that would be non-toxic to iPSCs and do not affect their pluripotency
- Designing an antibiotic selection-free approach to generate gene knockouts since drug selection usually leads to unexpected genome insertions
- Reducing the time taken to generate gene knockouts without compromising on iPSC transfection efficiency
The Solution
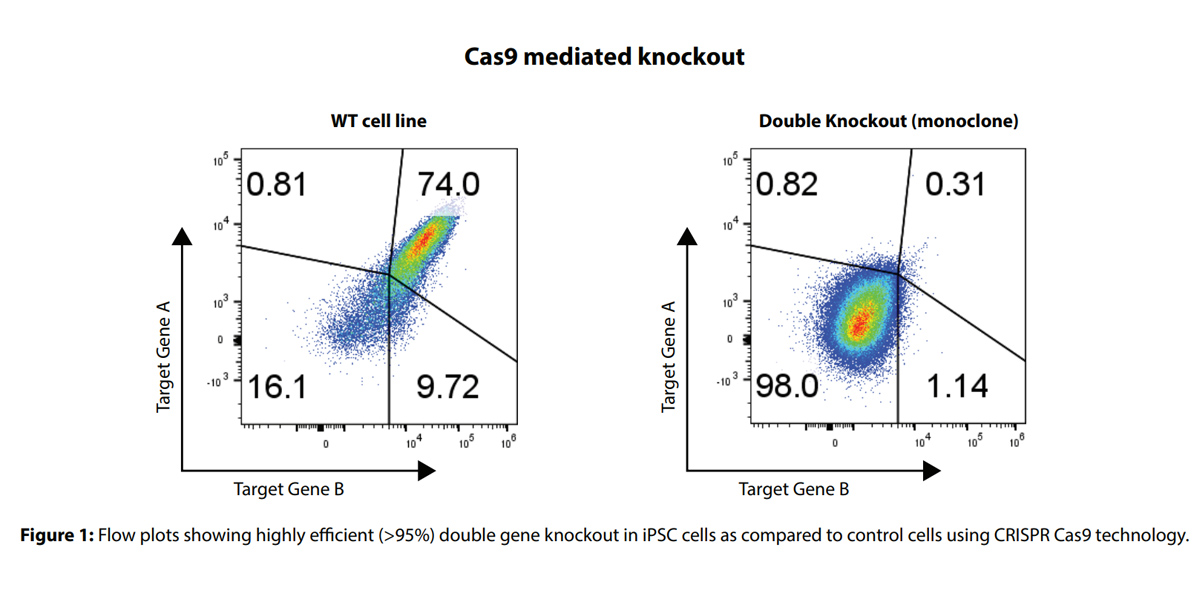
Syngene is equipped with several electroporators (4D nucleofector and neon transfection system, to name a few) for optimizing transfection efficiency in iPSC cells. We initially optimized CRISPR knockout efficiency in iPSC cells to obtain >50% knockout efficiency using Cas9 technology. The knockout bulk pool cells were FACS (fluorescence-activated cell sorted) sorted into single cells, and plated in multiwell plates. Later, the single cells were expanded to form individual colonies. We then successfully generated single-cell monoclones of gene knockouts in multiple iPSC lines (Figure 1).
The knockout clones generated were highly pure. We used them to establish a reproducible protocol to generate robust knockouts in several iPSC lines in 12-16 weeks.
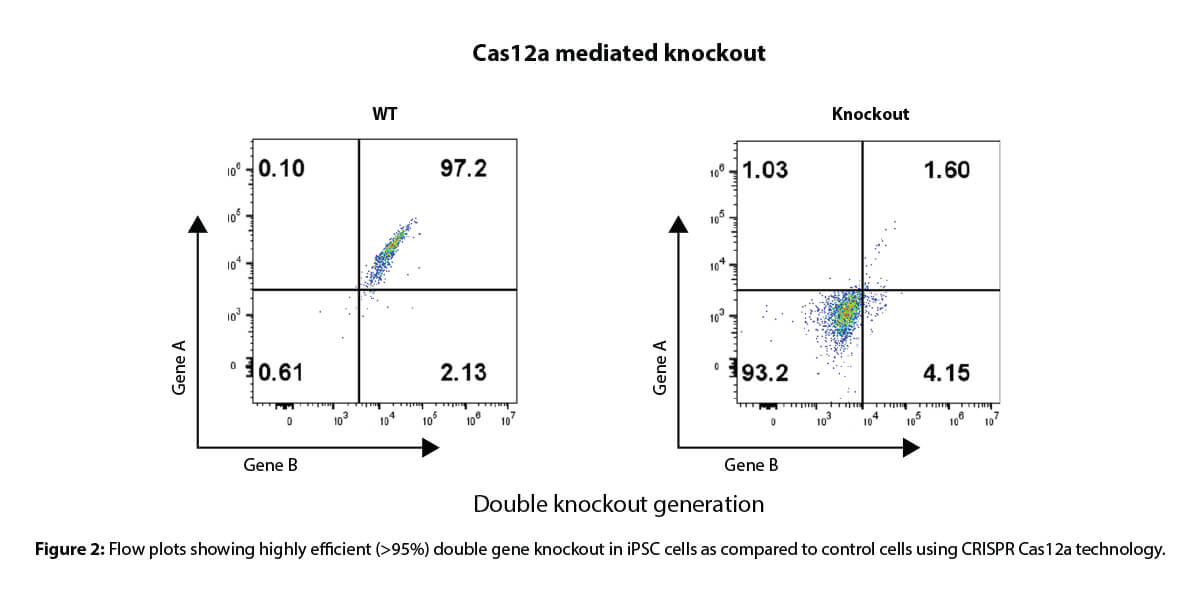
At this stage, the client expressed a desire to reduce the time taken to generate gene knockouts by 2-3 fold. To accommodate this request, we generated requisite iPSc knockouts in five weeks using Cas12a-mediated CRISPR gene knockout technology. This was accomplished with high transfection efficiency of >95% without the need for FACS sorting (Figure 2).
We achieved this while ensuring high transfection efficiency and stability of clones and at optimum costs. The client was delighted with the quality of the output as it helped them take their research to the next level for an iPSC-based allogenic gene therapy.
Conclusion
After completing this phase of the work, the client continued to work with us to establish the use of other advanced nucleases like Mad7 to generate knockouts in iPSC lines.
Syngene not only has expertise in generating gene knockouts in human iPSCs for allogeneic cell and gene therapy, we also provide geneediting services in iPSC cells. This involves gene knockout and knockin for disease modeling, allogenic therapies, and reporter cell line generation for drug screening. Further, we have expertise in generating CAR-iPSCs for CAR-T cell therapy. Our CRISPR package includes gRNA designing, KO generation, validation, and assays.
To know more about our IPSC services, contact our experts.