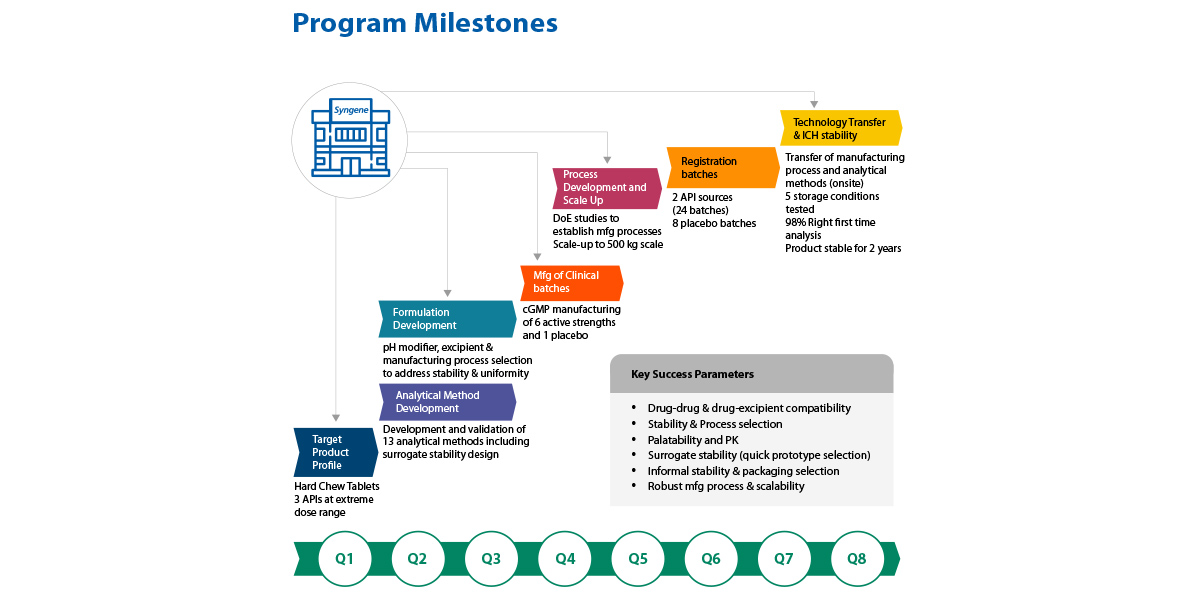
Business Problem
One of our leading multi-national animal healthcare clients was working on a novel three-drug combination product for the treatment of seasonal ticks in companion animals.
The client wanted to stabilise a low dose drug which is highly prone to hydrolytic degradation, and also ensure content uniformity of the active within the microgram dosage. The problem was unique and challenging, as the tabletting adjuvants were incompatible, and required our team to think outside the box to develop a stable formulation composition.
Key Program Features | Benefits |
|---|---|
Scientific capability |
|
Process improvements |
|
Result
- Syngene partnered with the client to deliver a first-in-class, complex 3-drug combination dosage form.
- Successfully completed screening of different prototypes considering chemical and physical stability, palatability, processability and drug dissolution.
- Performed seamless technology transfer to commercial scale (granulation lot size of 500kg) by adopting scale-up factors, performing risk assessments, identifying critical process parameters (CPPs) and designing appropriate control strategies.