At a glance
For a small-cell lung cancer (SCLC) program using a half-life extended bispecific antibody, Syngene
transferred and validated Meso Scale Discovery (MSD) platform-based ligand-binding assays for
pharmacokinetics and immunogenicity. The pharmacokinetics assay reached high sensitivity in a
direct serum matrix, while the immunogenicity assay delivered robust ADA detection even in the
presence of circulating drug. Automated preparation of calibrators and controls on a Tecan system
reduced variability across large clinical batches. Together, these bioanalysis workflows provided
advanced bioanalytical laboratory solutions for biologics and supported submission-ready clinical
data for a complex oncology asset.
Background: pharmacokinetics and immunogenicity in bispecific antibodies
The sponsor was developing a bispecific antibody with one arm targeting a tumor-associated antigen and the other engaging the CD3 receptor on T cells. By bringing T cells and tumor cells into close proximity, the molecule was designed to drive a cytokine-mediated immune response against SCLC. The half-life extension built into the construct meant that drug levels could remain measurable over long dosing intervals, increasing the importance of precise pharmacokinetics profiling.
For such bispecific antibodies, regulators expect a clear understanding of both exposure and immunogenicity. The clinical program therefore required sensitive pharmacokinetics measurements and a reliable immunogenicity assay to detect anti-drug antibodies (ADA) over a wide range of drug concentrations. The sponsor selected Syngene to transfer, optimize, and validate MSD platform-based ligand-binding assays and to perform regulated bioanalysis for submission-supportive clinical studies.
Scientific and operational challenges in clinical bioanalysis
The project combined several scientific and operational challenges. The T cell–engaging design of the bispecific antibody created a risk that the circulating target could interfere with free-drug quantification. Clinical samples had to be analyzed directly in serum, without applying a fixed minimum required dilution, which placed more pressure on the pharmacokinetics method to remain stable across a wide concentration range.
At the same time, sponsor-developed methods needed to be transferred to the MSD platform and validated under relevant bioanalytical guidelines. Large late-stage studies generated substantial sample volumes, so calibrators and controls had to be prepared in bulk with consistent quality. Immunogenicity testing demanded a highly sensitive immunogenicity assay that could still function in the presence of high circulating drug. These combined factors meant that the pharmacokinetics and immunogenicity workflows had to be designed and executed in a way that tightly controlled variability while maintaining high specificity and sensitivity on the MSD platform.
MSD platform-based pharmacokinetics assay transfer and validation
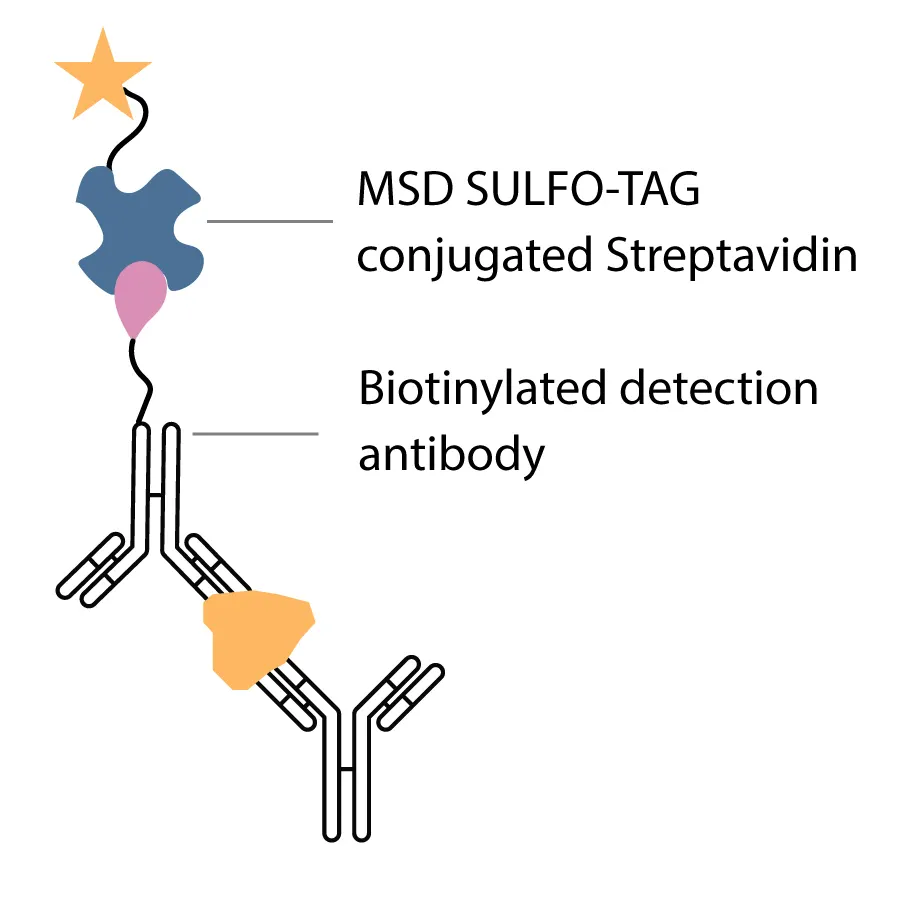
Syngene transferred the sponsor’s PK method for quantifying circulating bispecific antibody onto the MSD platform using a sulfo tag-based detection system. The assay was configured in a direct serum matrix format, so there was no predefined minimum required dilution. This was important for accurately capturing pharmacokinetics across early high-exposure time points and late low-level time points in a half-life extended molecule.
The transferred pharmacokinetics method used a biotinylated antibody in combination with sulfo tag–labeled streptavidin to generate a signal. The assay achieved an MSD platform sensitivity of 0.4 ng/mL, enabling detection of low concentrations near the tail end of the dosing interval. Specificity was confirmed in the presence of 1 ng/mL of soluble target, demonstrating that the assay could quantify free bispecific antibody without significant interference. The pharmacokinetics assay was validated for precision, accuracy, selectivity, stability, and dilution integrity under regulated conditions, confirming that it could support robust PK profiling throughout the clinical study.
Immunogenicity assay development on the MSD platform
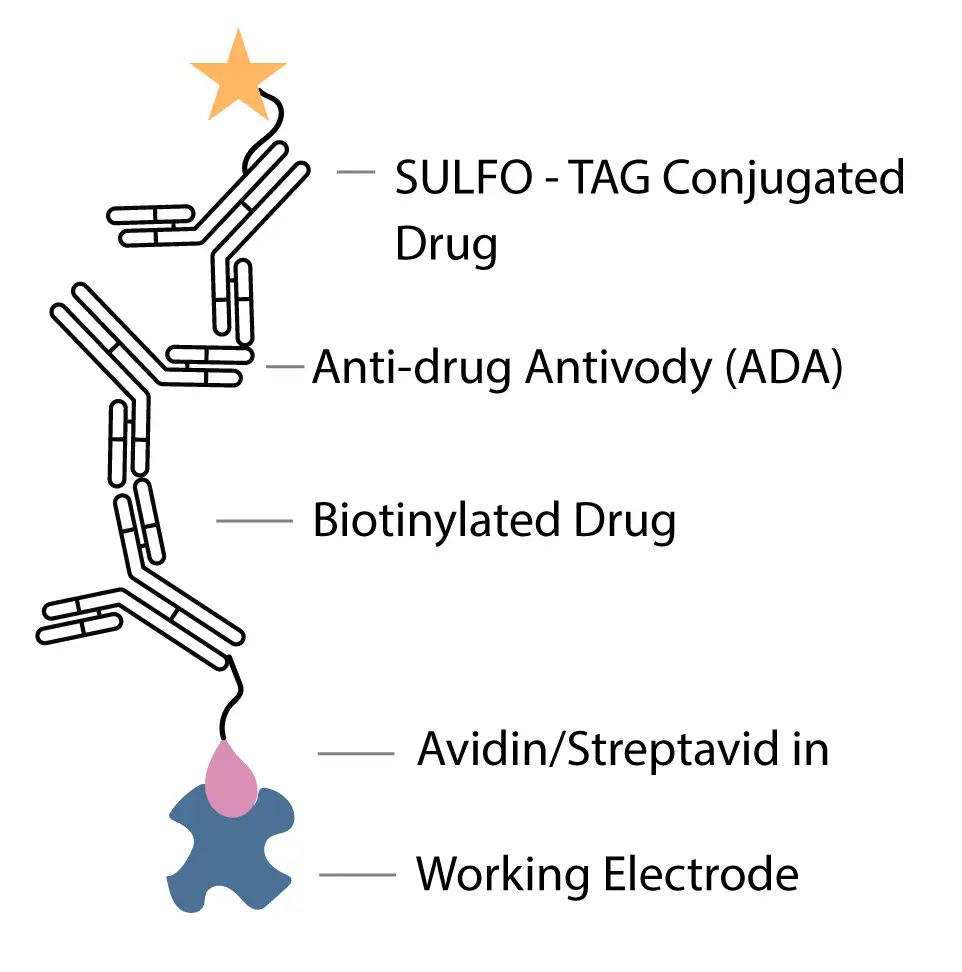
For immunogenicity, Syngene developed a bridging ELISA-type ligand-binding assay on the MSD platform to detect ADA against the bispecific antibody. The immunogenicity assay used streptavidin–sulfo tag–based detection in a master mix that contained both biotinylated and streptavidin-tagged forms of the drug. This configuration generated a highly specific signal for ADA while leveraging the sensitivity of the MSD platform.
The validated immunogenicity assay achieved a sensitivity of less than 5 ng/mL ADA. Drug tolerance studies showed that ADA concentrations from approximately 12 ng/mL up to 10,000 ng/mL could be measured in the presence of at least 10 µg/mL circulating drug. This performance allowed treatment-emergent ADA to be detected even when patients had high residual drug exposure, a common challenge with long-half-life bispecific antibodies. Overall, the immunogenicity method delivered a reliable view of ADA incidence and magnitude for the oncology program.
Controlling variability with automation in clinical bioanalysis
Because the pharmacokinetics assay used a direct serum matrix and did not apply a fixed minimum required dilution, each clinical run required multiple sets of calibrators and quality-control samples across the concentration range. Preparing these materials manually at scale could introduce lot-to-lot variability, increase operator burden, and threaten the consistency of pharmacokinetics and immunogenicity data over time.
To address this, Syngene implemented automated preparation of calibrators and controls using a Tecan liquid-handling platform. Automation standardised bulk preparation procedures, reduced operator-dependent variability, and improved reproducibility across clinical batches. For a submission-supportive study, where regulators closely review trends in pharmacokinetics and immunogenicity over the life of the trial, this automated bioanalysis workflow was a key contributor to data reliability.
Study operations, pharmacokinetics profiling, and immunogenicity readouts
Using the validated MSD platform methods, Syngene generated subject-wise concentration–time profiles for patients treated with the bispecific antibody. The high sensitivity of the pharmacokinetics assay allowed a detailed characterization of the exposure profile, including early peak levels, distribution phases, and terminal elimination behavior. These profiles supported estimation of key pharmacokinetics parameters such as Cmax, AUC, and half-life for use in clinical and regulatory decision-making.
Immunogenicity samples were tested using the MSD-based bridging immunogenicity assay. The broad drug tolerance range reduced the need for complex pre-treatment steps and helped simplify the interpretation of ADA incidence and titers. Assay performance remained stable across clinical batches, with no major technical issues observed during routine bioanalysis, which further strengthened confidence in the immunogenicity data package. All pharmacokinetics and immunogenicity outputs were generated in a GLP-regulated environment with appropriate documentation for regulatory submissions.
Outcome and sponsor value
The combined pharmacokinetics and immunogenicity strategy delivered several concrete advantages for the sponsor. High-sensitivity pharmacokinetics data from direct serum analysis, paired with a focused MSD platform immunogenicity assay, enabled a clear understanding of how the bispecific antibody behaved in the body and what immunogenicity risks might arise over time. Automated Tecan-based preparation of calibrators and controls reduced run-to-run variability and operator bias, leading to more consistent bioanalysis results across large clinical batches.
The methods transferred to the MSD platform were fully validated, well documented, and demonstrated stable performance over the duration of the study. This gave the sponsor confidence that both pharmacokinetics and immunogenicity data would withstand regulatory scrutiny. In addition, the same assays could be used for ongoing submission-supportive studies without major re-optimization, which helped protect clinical timelines. Overall, the work illustrated how a structured pharmacokinetics-led bioanalysis strategy can de-risk and streamline development of half-life extended bispecific antibodies in oncology.
Why Syngene for pharmacokinetics and immunogenicity in bispecific antibodies
Sponsors developing complex biologics such as bispecific antibodies often need partners who understand both assay science and the practical realities of clinical development. In this project, Syngene combined experience with bispecific antibodies and other novel large molecules, strong ligand-binding assay development skills, and deep familiarity with the MSD platform for pharmacokinetics and immunogenicity applications.
Teams with expertise in method transfer, optimization, and validation under relevant GxP guidelines worked alongside robust sample management and automation specialists to handle high clinical sample volumes. This combination of scientific depth and operational strength enabled the sponsor to generate high-quality pharmacokinetics and ADA data for a challenging oncology program, while retaining flexibility to support future study phases and regulatory interactions.
Syngene’s bioanalytical capabilities in pharmacokinetics and immunogenicity
Syngene’s advanced bioanalytical laboratory solutions for biologics provide end-to-end support for pharmacokinetics and immunogenicity assessment across large-molecule development programs. Dedicated laboratories handle bioanalysis of monoclonal antibodies, bispecific antibodies, nanobodies, fusion proteins, and other complex modalities. Teams design and execute fit-for-purpose and fully validated assays for PK, PD, ADA, neutralizing antibodies, and exploratory biomarkers using ligand-binding and related technologies on platforms including the MSD platform.
These bioanalysis services support studies from preclinical toxicology through first-in-human trials and late-stage clinical development, under GLP quality systems. Close integration with translational research and clinical development groups allows efficient sample handling, streamlined data workflows, and rapid troubleshooting when unexpected pharmacokinetics or immunogenicity profiles arise. Sponsors can partner with Syngene for regulated bioanalysis, translational research, and clinical trial support, helping to accelerate development timelines, improve decision-making, and build strong, submission-ready data packages for innovative biologic therapies.







