Benefits of outsourcing stability testing
- One of the key benefits of outsourcing is the access to infrastructure. Manufacturers do not have to acquire the equipment/ technology or invest in any of the assets, which might not be cost-effective in the long term. Instead, they can choose to outsource, where they also get the benefit of the expertise from which cost-effective solutions can arrive within an agreed time frame.
- Sponsors can focus on product development, quality, and commercialization by outsourcing stability.
- CROs cater to clients across multiple locations and time zones and effectively manage communication, thereby offering flexibility in collaboration. This minimizes the need for oversight as the CRO-client relationship matures.
Introducing Baxter
Baxter International is one of the leading manufacturers of intravenous (IV) fluids and systems. Being a specialist in medical products. It also has an extensive range, including infusion pumps, pre-filled syringes, biological sealants, and inhaled anesthetics, as well as dialyzers and other products for the treatment of end-stage renal disease (ESRD). Founded in 1931 as an intravenous products maker, Baxter has come a long way in terms of business growth and its offerings.
Factors considered by Baxter while outsourcing stability testing to Syngene
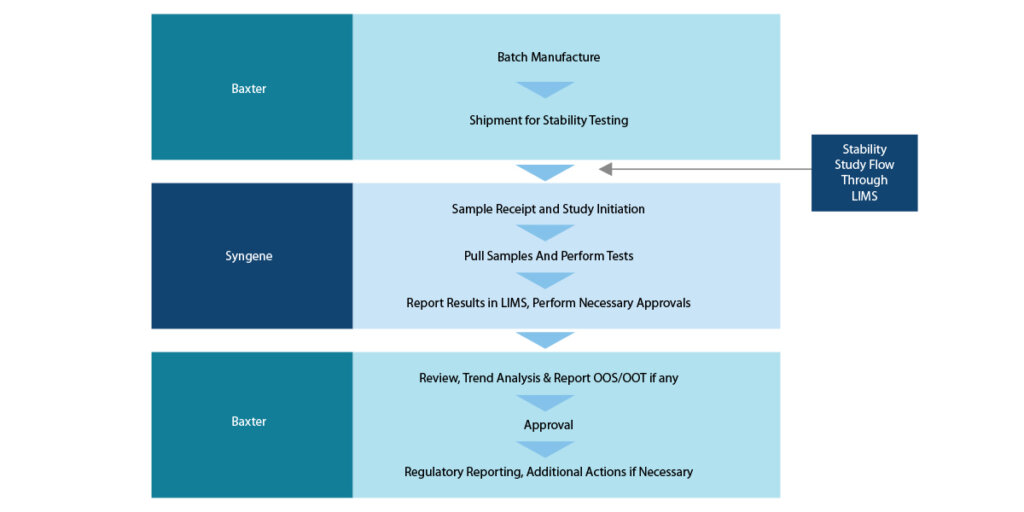
In 2013, Baxter International Inc. collaborated with Syngene International Ltd. to establish Baxter Global Research Centre (BGRC) for its stability study operations. Baxter did a comprehensive review of several factors to understand the capabilities of prospective CROs and to gauge how successful the collaboration would prove to be. The collaboration established a customized stability program for Baxter’s products with a dedicated team of experts and adequate infrastructure to ensure result-oriented processes. The core team consisted of scientists who had in-depth knowledge and rich experience. Advanced training was provided to ensure the confidentiality and integrity of the operations carried out for Baxter.
While Baxter’s focus at this point of time was on R&D activities centered on product and analytical development and pre-clinical evaluation, this publication will focus on stability operations. This phase of decision-making was extremely crucial for Baxter as the CRO to be selected was to be able to professionally deliver the objectives to an optimum level so that the facilities would be made complete use of, thus making it a pragmatic investment for the company.
Before defining and arriving at the processes and the expected results, knowing the scope was important. The below mentioned scope of business was framed after understanding Syngene’s abilities and Syngene agreeing to feasible deliverables.
Analytical and allied services
Stability Studies | Multiple batches across product families annually |
|---|---|
Reference Standard
Management | Synthesis, Qualification/Characterization, Stability studies to support Storage and Distribution |
Analytical | Method development, Validation and Life cycle management of analytical methods |
Other | Analytical testing, Evaluating & verification of analytical methods for compendial proposals, assessment of elemental impurities |
Following were the attributes that were considered during the selection and decision-making process.
With approximately 72,000 sq. ft. of facility space, Syngene stability programs support small and large molecules. The campus has multiple walk-in and reach-in chambers and modular labs customized to suit the customer’s requirements.
A critical need for the stability program was the storage chambers. A detailed prediction of the number of batches to be supported per year was needed, along with the planned storage conditions. This helped Baxter and Syngene accurately calculate the storage capacity needs. When planning, it was recommended to also consider building a slight excess in capacity for unexpected peaks in demand or future growth. These factors were thoroughly worked upon to conclude that Syngene had the needed capabilities, either in existing infrastructure or in space to build, to make this collaboration profitable and sustain growth.
Governance Processes
After articulating the requirements and identifying the attributes of possible requirements, the implementation process started, which took about seven months. Baxter and Syngene also designed operational excellence measures for those elements that would be involved in ensuring a smooth workflow.
To ensure operational excellence, Baxter categorised the activities to track and enhance the deliverables. The operational excellence is measured with realistic metrics that gauge the performance of the attribute respectively.
Attribute | Item | Performance indicators |
|---|---|---|
Delivery Timeliness | On Time SOW Completion | NLT xx% |
Delivery Quality | Average Review Iterations First time right | NMT x NLT xx% |
Logistics | Client Shipments
(Pick up to Arrival) Import Licenses Chemical Procurement | X days |
A robust quality agreement was essential to ensure clarity in expectations of each function as well as define decision making authority. Overall the governance process ensures effective tactical execution, yet allows for expansion into strategic adjacencies which were identified by both Baxter and Syngene.
Activity | Details |
|---|---|
KPI Tracking | • Defects/ Deliverable with Categories • Documents Review; Average Iterations/Doc & Turn around time |
Logistics Metrics | • Licenses, Procurement & Shipment Turn around time |
Deliverables | • Meeting Timelines and Business Targets |
Lean/ Six Sigma Projects | • Identify Key Business Goals / Processes to Improve • Six Sigma DMAIC approach / Lean Methodology |
Productivity Improvment | • Continuous Improvement Initiatives • Knowledge Management |
Conclusion
The Baxter-Syngene collaboration makes it evident that for a successful strategic partnership, clear communication and alignment of resources to fulfil mutual objectives are very important. Baxter stated that knowledge-sharing and implementation of defined processes and tools (including governance structure for issue escalation) enabled the scientific rigor and regulatory compliance. This ensured the successful implementation of this collaborative stability testing program. Baxter also identified that a clear communication plan is one of the elements of a successful collaboration. Well-defined objectives, robust training and adequate knowledge transfer are crucial which were followed with proper metrics, readiness planning and regular communication to understand the progress of ongoing work.