Inside the growing precision therapy market and the crucial role of pDNA manufacturing

Challenges in pDNA manufacturing for precision therapies, and how CDMOs, who cater to the requirements of multiple companies and develop platform processes to achieve the much-needed economy of scale, are being seen as a possible solution.
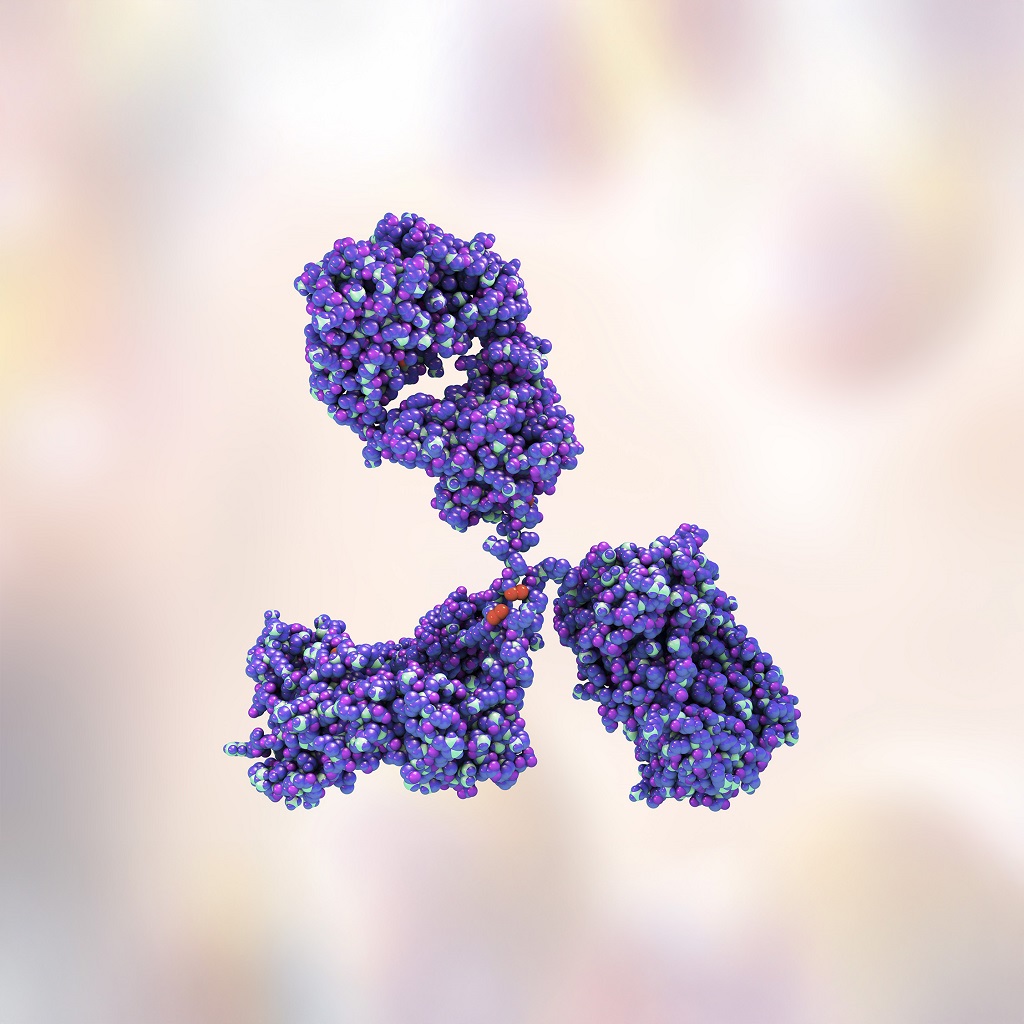
Manufacturing the drug substance for Zoetis’ first-in-class mAb osteoarthritis drug

Learn how Syngene supported leading animal health company Zoetis, manufacture the drug substance for Librela® (bedinvetmab), a first-in-class monoclonal antibody, to treat osteoarthritis in dogs.
How Syngene supported a biotech company in developing a bile acid modulator for treating cholestatic diseases

Learn how Syngene successfully identified a first-in-class, potent, and efficacious inhibitor for the apical sodium-dependent bile acid transporter and sodium/taurocholate co-transporting polypeptide targets for treating cholestatic diseases.
Harnessing artificial intelligence and digital technologies across the biopharma value chain

Key considerations when using AI, and how Syngene applies AI and digital technologies to accelerate drug discovery, development, and manufacturing for its clients.
ADCs as biological missiles for targeted therapies

Explore the market growth of ADCs, innovations in the ADC space, and the future of ADCs as a targeted therapy for various diseases, including cancer.
Antibody Drug Conjugates: Re-emergence of an old modality for targeted therapies
Explore the key challenges in ADC development and how Syngene, a leading CRO/CDMO, is supporting clients in the discovery, development, and manufacturing of innovative ADC molecules.
How Syngene enabled an emerging pharma company to develop an innovative drug to treat erectile dysfunction

Learn how Syngene transitioned a drug meant for treating erectile dysfunction from late-phase discovery to early-phase clinical studies for an emerging pharma company.
How to overcome solubility challenges by applying amorphous solid dispersion: An end-to-end solution

Discover the successful approach to preparing, screening, characterizing, and dosing amorphous solid dispersions (ASDs) in preclinical and early clinical development.
Evaluating bioequivalence of an injectable for Type-2 diabetes and weight management

Learn how Syngene supported a global pharma company in conducting human pharmacological studies for Semglutide as an injectable while overcoming clinical and analytical challenges.
Xenografted tumors share comparable fraction unbound and can be surrogated by mouse lung tissue
Publication: Drug Metabolism and Disposition






