How Syngene supported a biotech in developing a PROTAC molecule for treating cancer

Learn how Syngene successfully developed and manufactured several kilograms of a PROTAC molecule under cGMP conditions within 12 months for treating a certain type of cancer.
Discovery Services

Syngene offers integrated and standalone services across the drug discovery continuum from Target Identification to IND enabling.
Harnessing the power of induced proximity-based drugs and precision medicine for treating cancer

How Syngene is supporting clients in treating cancer using targeted protein degradation, protein stabilization approaches, and precision medicine therapies.
Viral clearance from conventional to unconventional process steps in Biologics
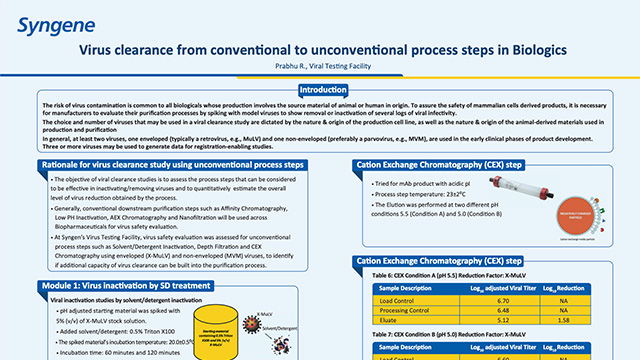
Learn how viral clearance from unconventional steps can add value to the total viral clearance independently or along with conventional process steps.
How Syngene supported a clinical-stage oncology company in manufacturing the RSM for treating a rare tumour

Learn how Syngene reduced the number of steps required to synthesize an RSM for two API candidates by 50% and successfully manufactured and supplied the GMP product to the client.
Detection and quantitation of process-related impurities in biopharma manufacturing

Learn the challenges in the detection and quantitation of process-related impurities, including nitrosamine and azido impurities, and Syngene’s capabilities and solutions to resolve them.
Holistic, mechanism-driven approach to toxicity assessment using Computational and Data Sciences

Learn how Syngene provides in silico and in vitro support for holistic toxicity assessment using Computational & Data Sciences.
Development and Manufacturing services

Syngene’s offers an end-to-end platform to deliver integrated and standalone services for small molecules across the Development continuum.
Characterization of acquired resistance mutations to menin inhibitors
Publication: American Association for Cancer Research
LP-07 In silico analysis using Derek and Sarah along with ICH M7 expert review accurately predicts Mutagenicity and Carcinogenicity of Nitrosamines
Publication: Toxicology Letters






