Partnering for Precision in Small Molecule Drug Substance Development & Manufacturing

Read how Syngene accelerates small‑molecule development with integrated discovery‑to‑commercial capabilities and globally compliant GMP facilities. Explore how advanced process chemistry, analytical depth, and seamless tech transfer de‑risk scale‑up and strengthen manufacturing reliability.
Engineering the Future of Chemistry- New Modalities in Action: Expanding the CRDMO Frontier

Discover how our next‑generation modalities—photochemistry, biocatalysis, electrochemistry, and flow—unlock cleaner pathways and overcome synthetic bottlenecks. Read how these technologies enable faster optimization, late‑stage functionalization, and scalable, sustainable chemistry. Explore how our integrated platforms accelerate innovation from early discovery to development.
Redefining Boundaries in Sustainable Metathesis Chemistry

Explore how Syngene advances Ring‑Closing Metathesis with sustainable, catalyst‑efficient processes that boost yields and minimize waste. Learn how our ethylene‑trapping and recycling strategies reduce environmental impact while strengthening process robustness. See how scalable, green metathesis solutions can elevate performance across your development and manufacturing workflows.
Bioanalysis for a fit-for-purpose PK assay for a novel bispecific nanobody

Discover how Syngene developed a fit-for-purpose PK assay for a novel bispecific nanobody, achieving a 1.00 to 1000 ng/mL dynamic range and a low 1% repeat rate to support clinical dose selection.
Automation driven optimization of liver microsomal stability (LMS) assays for ADME studies

Syngene enhances ADME studies with automated liver microsomal stability assays, improving DMPK precision, boosting throughput, and accelerating ADME screening workflows.
Intraperitoneal Injection of Sesame Oil as Vehicle Induces Decidual Reaction in Female Rats: A Review of In-House Data
Publication: Sage Journal
The Future of Animal Health: How Integrated Small and Large Molecule Innovation Is Transforming Veterinary Therapeutics

Integrated cell line development and biologics development are reshaping veterinary therapeutics. Discover how multi-modality innovation drives impact—read more today.
Bile acid modulators in discovery: Identifying cholestasis candidates with the best chance of success
Publication: Pharmaceutical Technology Focus
India’s only GLP-certified viral testing and clearance services
Syngene has India’s only GLP-certified viral testing facility that offers comprehensive viral safety solutions for your biologics program. Whether it’s studies supporting applications for Phase I, Phase III, or marketing authorizations, we have consistently delivered them with responsibility and unwavering commitment. At Syngene, viral safety is non-negotiable. Delivering safe and high-quality drugs to patients is […]
The rise of ADCs in oncology: how antibody drug conjugate therapies deliver precision
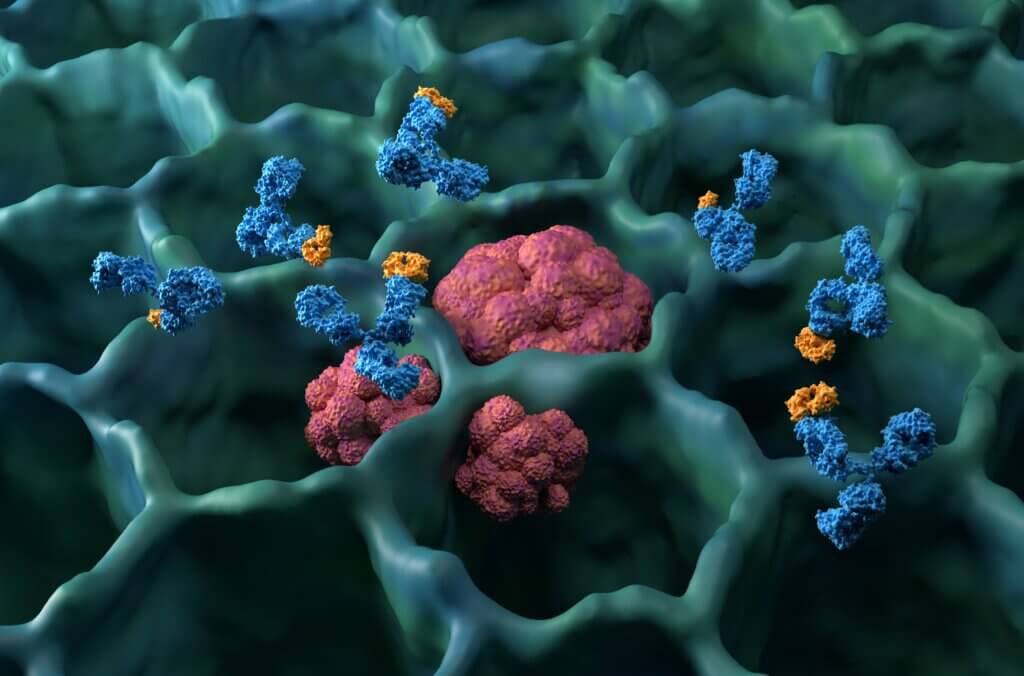
Understand antibody drug conjugate science, bioconjugation, and manufacturing controls that shape targeted therapy for cancer. Read the article today.






