Effect of Container Closure Systems on Stability of Pharmaceutical Products
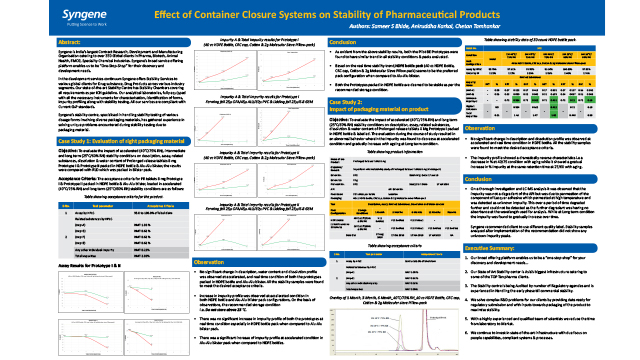
Case studies on how Syngene handled unique problems in Stability Testing arising from packaging material.
Efficient Scale Up of Therapeutic Antibody Manufacturing Processes

Syngene’s ‘first-time-right’ Technology Transfer approach to achieve timely clinical and commercial milestones.
Mechanistic insights into drug-drug interactions from large-scale toxicogenomics data

How to manage the risk of drug-drug interactions in drug development and during poly therapy especially with elderly patients.
Profiling CEACAM7 as a potential target for pancreatic cancer
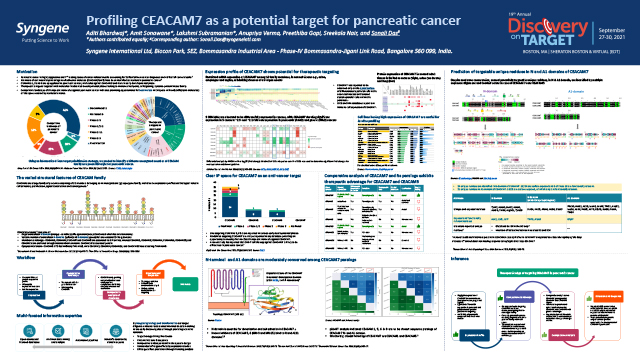
Demonstrating the therapeutic edge of targeting CEACAM7 in pancreatic cancer using an integrative approach between biology and bioinformatics.
A quick, cost-effective process for large-scale compound synthesis for a leading pharma company

How Syngene achieved cost minimization and quick turn-around time when scaling a client’s drug compound from lab-scale to cGMP-scale using the ‘SELECT’ criteria.
Hyaluronic Acid – A wonder molecule for the cosmetic and pharma industries

Understanding the many application areas of Hyaluronic acid in the cosmetic and pharma space, including possibilities for the future.
How collaboration with Syngene helped a global biopharma fast-track novel molecule development for global licensing
Learn how a partnership with a leading global biopharma for outsourced Bioanalytical studies for Large Molecule therapeutics evolved into a co-development partnership nearing a decade.
Design of Experiment (DoE) – An efficient tool for process optimization
DoE is achieving increasing acceptance in the pharmaceutical sector as it is being seen as an efficient tool for process optimization, including helping to develop quality products.
Things you may not know about continuous flow chemistry
The continuous flow chemistry processes established in the lab can now be readily transferred to the production facilities and scaled up for commercial use without substantially altering reaction conditions.
Manufacturing vaccines during COVID-19
How and why CROs/CDMOs are better positioned to bridge the gap between vaccine demand and supply, safely and at speed.






