Urban Mental Health: Evidence, Action, and Impact through BUMHI

Explore how the BUMHI initiative advances urban mental health through research, evidence-based action, and community impact to build healthier cities.
Cost of Goods Reduction by Design: Strategies for Early-Phase Process Optimization

Reduce CoG by using design of experiments, quality by design, and smart tech transfer in early development. Practical strategies to cut cost without risk.
Computational toxicology – The new frontier in predictive safety assessment

Peptide synthesis and the hidden complexities of scaling peptide therapeutics, from SPPS to purification, analytics, and cGMP manufacturing. Talk to our team today.
Project Optimus – Transforming the paradigm of dose optimization and selection in oncology

Discover how Project Optimus is changing oncology drug development through data-driven dose optimization and modeling strategies. Learn more today.
Peptide synthesis and the hidden complexities of scaling peptide therapeutics

Peptide synthesis and the hidden complexities of scaling peptide therapeutics, from SPPS to purification, analytics, and cGMP manufacturing. Talk to our team today.
Hybrid, device-enabled IPF cough trial for a Swedish sponsor— integrated formulation-to-site execution by Syngene

In this case study, you would learn how Syngene enabled a hybrid, device-based IPF cough trial in India for a clinical-stage pharma company—delivering seamless formulation-to-site execution with robust data integrity, regulatory navigation, and on-time delivery.
How to choose a CRDMO at CPHI Frankfurt 2025: A Fieldguide

CPHI Frankfurt 2025 is the year’s most efficient place to compare science, scale, and reliability in one hall. Sponsors meet teams, see facilities represented across regions, and validate claims in real time.
Syngene Enables Orocidin to Achieve Clinically Acceptable Antimicrobial Peptide

OROCIDIN A/S, a company focused on developing a novel treatment for advanced gum disease, or aggressive periodontitis, partnered with Syngene for the synthesis of their lead molecule
Advancing mAb manufacturing with customer-centric solutions
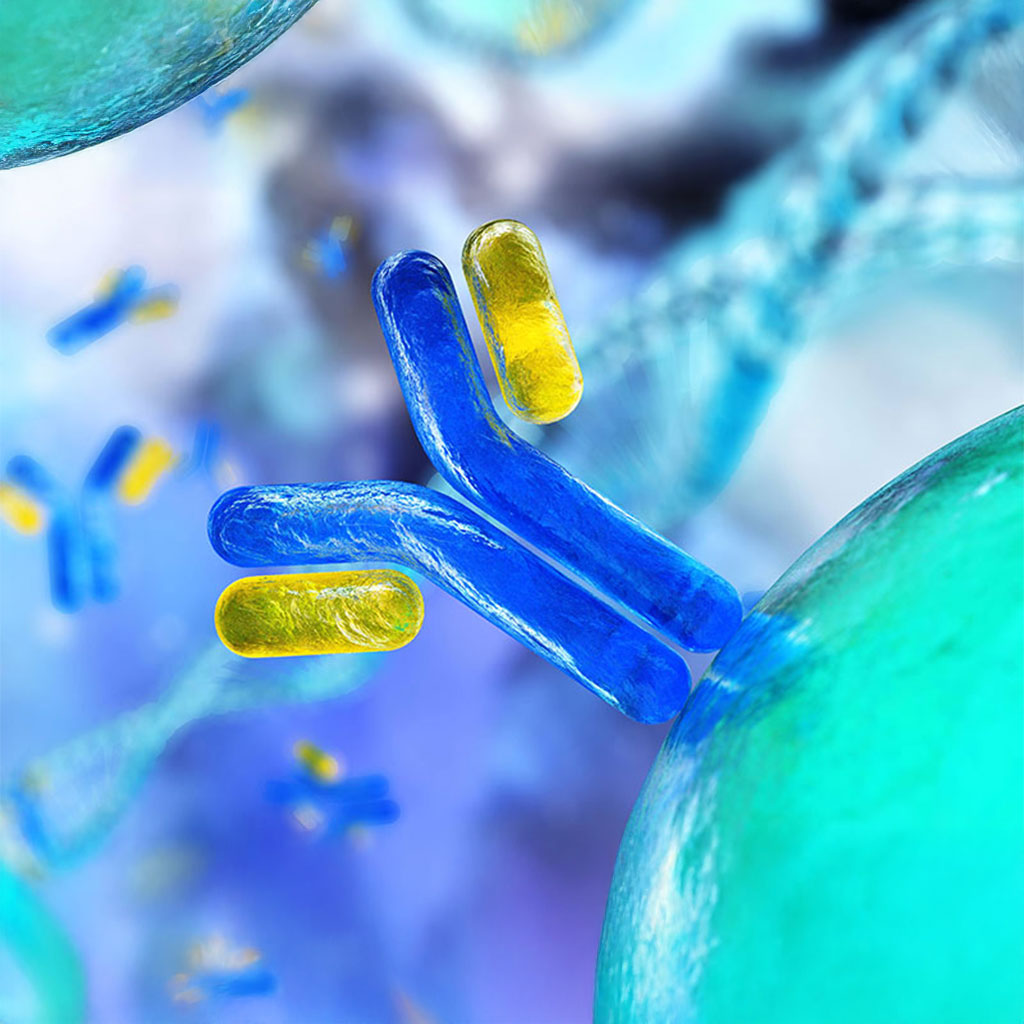
Monoclonal antibodies (mAbs) are lab-engineered proteins that mimic the ability of the immune system to neutralize harmful pathogens. Produced by identical immune cells cloned from a single parent cell, these antibodies target a specific antigen with high precision. Their applications span across medicine, treating cancers, autoimmune disorders, and infectious diseases, as well as diagnostics and research.
Rise of the Indian CRO-CDMO: From Cost Arbitrage to Innovation Leadership

From ‘Pharmacy of the World’ to becoming the preferred outsourcing destination for innovative scientific solutions, India’s Life Science industry has seen a dramatic shift. Driving this change is the contract research development manufacturing organization (CRDMO) industry, which witnessed a CAGR of 15% between 2019 and 24, double the global growth rate of 7%–8%.






