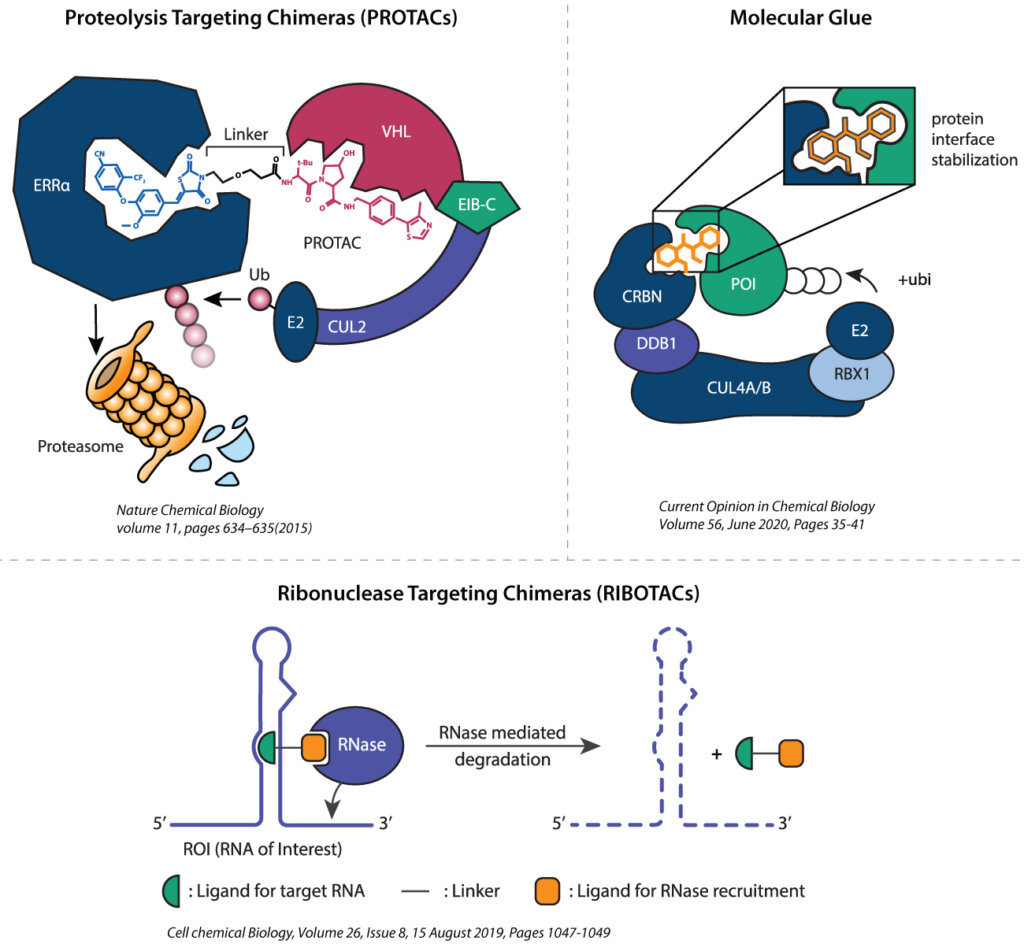
Introduction
PROTACs are heterobifunctional molecules comprising a ligand targeting a protein of interest (POI) and a ligand targeting an E3 ligase with a connecting linker. This novel design utilizes the organism’s own ubiquitin-proteasome system and induces proximity between E3 ligases and POI, leading to its proteasomal degradation. PROTACs, differ from conventional small molecules in their catalytic mechanism by way of inducing the degradation of the target protein. PROTACs have demonstrated success and remarkable biological responses across challenging targets, including non-druggable targets.
Not only is this novel strategy enabling the development of more potent and selective treatments for cancer and other intractable diseases, but it is also unlocking parts of the proteome that have traditionally been considered “undruggable”. As a result, targeted protein degradation (TPD) therapeutics have emerged as a relatively new modality with the potential to revolutionize the field of drug discovery.
With 200+ dedicated TPD scientists and more than 15 global clients, Syngene has a strong track record in accelerating PROTAC programs for its clients. To date, Syngene’s Discovery team has successfully identified Hits and leads across different projects, including programs moving into preclinical development through a strong collaborative effort (Figure 1).
Key challenges in developing novel degraders for TPD
Heterobifunctional degraders such as PROTACs offer a number of advantages over traditional small molecules. These include:
- Providing a step-up in regulating protein levels as PTOTACS can also affect the non-enzymatic function of the protein
- Providing an alternative to targeting drug-resistant proteins for overcoming tumor drug resistance,
- Effective against “undruggable” targets such as transcription factors
- Highly efficient against scaffolding molecules which cannot be targeted by the traditional small molecule inhibitors
However, several aspects need to be considered before developing these degraders and translating them from bench to clinic. Only a careful analysis will ensure that the degrader is efficient, specific, and optimized for its target protein (Figure 2)
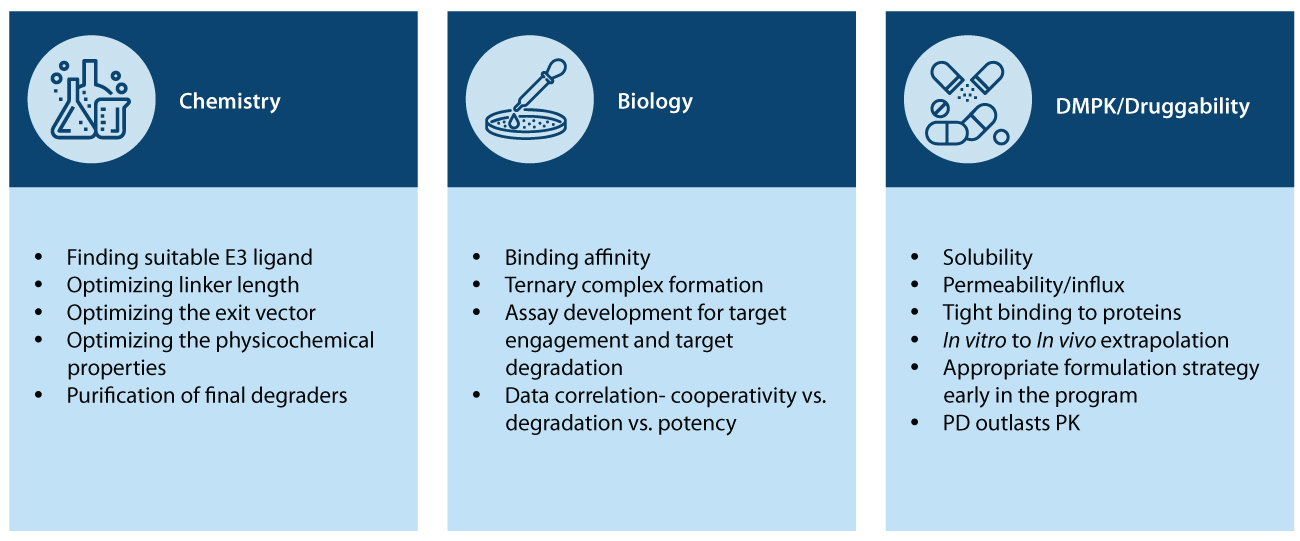
Although PROTACs are considered revolutionary, to date, the design and optimization of PROTACs remain empirical. This is due to the complicated mechanism of induced protein degradation and molecular weight and size, leading to solubility, permeability, and bioavailability issues. This limitation calls for an efficient workflow with assay development that helps enhance our understanding of the structure-activity relationship facilitating the rational design of PROTACs.
Heterobifunctional degraders consist of two different subunits performing distinct functions. Several properties need to be standardized for synthesizing a heterobifunctional molecule before it can be made to effectively induce the degradation of the target protein.
Mammalian cells consist of around 600 different ubiquitin ligase proteins, all suited to bind different proteins. The initial chemistry challenge to designing a degrader molecule is to choose a suitable E3 ubiquitin ligase and a stably binding exit vector. The two motifs then need to be attached using a linker of appropriate length so that the target protein and the E3 ligase are at the optimum orientation for ubiquitination.
Once such a degrader is designed, its physicochemical properties must be tested to ensure the maximum degrading effect, followed by its synthesis and purification for application to biological systems.
The success of the degrader in biological systems must be assessed on the following grounds:
Binding affinity to target protein in vivo
Formation of a stable ternary complex
Efficient engagement of target protein
Absence of non-specific binding
Potent degradation of the protein of interest
Finally, the potential to use the degrader as a therapeutic drug, or its ‘druggability,’ must be analyzed, based on the following:
Solubility in an aqueous medium
Permeability into mammalian, especially human cells
Stability and ability to bind tightly to the target protein
Pharmacodynamic (PD) and pharmacokinetic (PK) correlations
A heterobifunctional molecule that ticks all these boxes will have strong translational potential and clinical applications.
Syngene’s capability in developing degraders
A concerted approach to designing PROTACs that consider all the above challenges is the need of the hour. TPD strategies that can be used to tackle the growing number of undruggable targets are crucial for coming up with novel therapeutic approaches for treating real-world patients.
Syngene’s PROTAC capabilities put it at the forefront of developing protein degraders across a range of diseases. Syngene has been working right from establishing the proof-of-concept (POC) studies for the target of interest to hit identification and lead optimization until the nomination of a clinical candidate for TPD. We have supported our clients through our collaborative programs from early discovery to development and candidate selection for multiple targets in this segment.
Syngene is expanding its capabilities to support programs based on molecular glues, ribonuclease targeting chimeras (RIBOTACs), and lysosome-targeting chimeras (LYTACs), generally termed as X-TACs. Our experience in chemistry, biology, and DMPK allow us to create a roadmap for X-TACs research programs.
We have expertise in the following:
Hit identification and Targeted library synthesis
Computational drug designs for X-TACs
Synthesis, optimization, and purification
Screening and target degradation assay
DMPK characterization
Assessment of druggability
Hit identification and Targeted library synthesis
Syngene can offer top-of-the-line PROTAC chemistry allowing fast-track validation and proof of concept. We can do this by identifying PROTACs for targets through a rapid synthesis of the libraries, spanning small molecule binders with different exit vectors, linkers, and E3 ligands. This includes cereblon (CRBN), von Hippel–Lindau ligase (VHL), and inhibitors of apoptosis protein (IAP).
To date, Syngene has completed the synthesis of a large number of libraries with a very high success rate (>99%). Each library consists of a diverse set of linkers, exit vectors, and E3 ligands, which are processed through rapid purification using automated systems.
Computational Drug Designs for X-TACs
We provide computational drug design solutions for X-TACs by establishing the binding mode of warhead/ligand in the target protein, identifying exit vectors for attachment of linkers, and enabling library enumeration of target compounds with appropriate linkers.
Following the molecular dynamics simulations, the molecules’ functionality is tested by generating initial models of the ternary complex of target protein, ligand, and E3 ligase. Additionally, the insights from molecular simulations of the ternary complex provide a good understanding of the binding modes of ligands.
Medicinal Chemistry and Optimization
Syngene has strong expertise in X-TAC synthesis and optimization for TPD programs. We offer solutions that aid in the standardization of suitable E3 ligand, linker length, exit vector, physicochemical, and ADME (absorption, distribution, metabolism, and elimination) properties of the assembled molecules.
We have expertise in synthesizing linker acids and amines with E3 ligases in multigram quantities and PROTAC degraders on a scale for advanced studies. Our experience in this class of molecules gives us an advantage in providing clients with services from concept to preclinical candidates.
We have successfully optimized the different regions of a novel small molecule inhibitor and achieved sub-nanomolar potency with excellent metabolic stability and PK profile. We have also generated and modified various E3 ligase ligands such as CRBN, VHL, IAP, and MDM2 (mouse double minute 2 homolog) as part of our discovery efforts (Figure 3). Using these ligands, we have synthesized and optimized different PROTAC degraders with specific E3 ligands.
Biology
Despite rapid progress, PROTAC discovery remains challenging and laborious, and largely defined by an empirical process. Hence, it is important to have a set of techniques and assays that can characterize and rank such heterobifunctional compounds rapidly and efficiently for different properties and provide valuable feedback to guide medicinal chemists during the lead discovery and lead optimization process. The compound profiling for PROTACs involves a step-wise approach engaging the ubiquitin-proteasome degradation pathway using biochemical, biophysical, and cellular assays. This is to ensure that the molecules are not only penetrating the cells but also forming a ternary complex amongst POI, PROTAC, and E3 Ligase followed by target ubiquitination degradation (Figure 4).
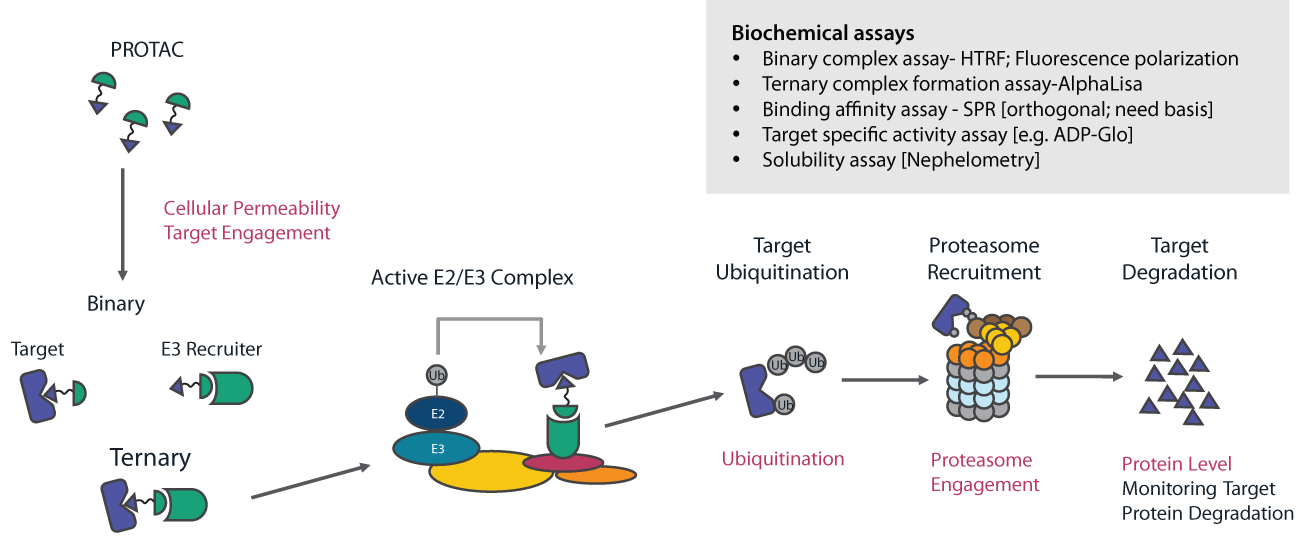
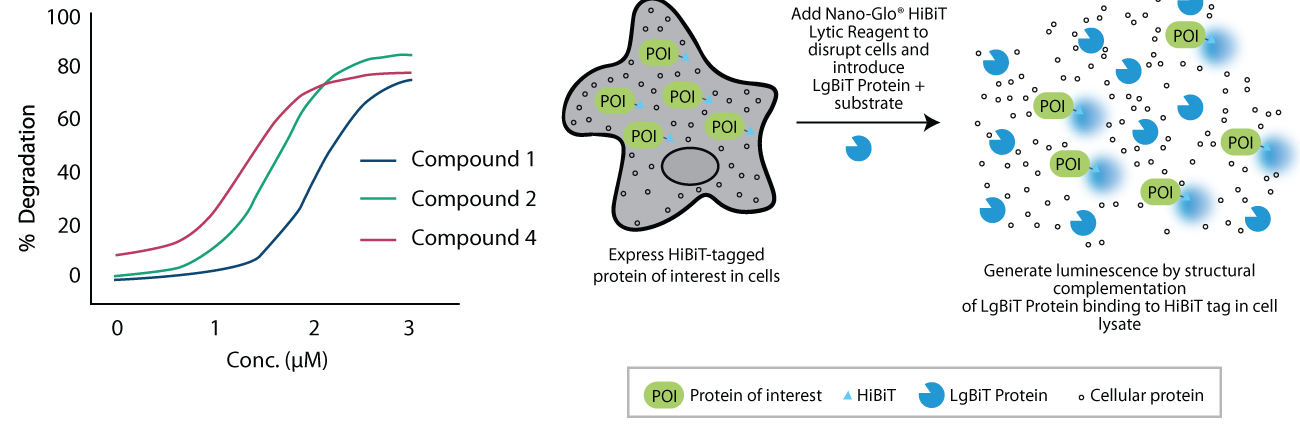
The Syngene Biology team has developed fluorescence polarization (FP), surface plasma resonance (SPR), and amplified luminescent proximity homogeneous assay (ALPHA) technology-based binding assays in the cell-free system for binding affinity characterizations. Additionally, our Assay Biology team has provided support in developing a cell-based target engagement assay using Nano-BRET and an inhouse derived ALPHA-LISA platform for determining the binding of compounds to E3 ligases. The target degradation in the cellular setting is further evaluated using different approaches, including regular Western blot, WES, HiBiT assay, and using In-Cell Western (ICW). HiBiT assay is a sensitive, faster, and high throughput assay using Nano-Glo technology and is extensively used for studying the targeted protein degradation intracellularly. We are currently conducting HiBiT assays for more than 15 targets in cell lines supplied by a partner or generated at Syngene (Figure 5).
The combination of these technologies and selection of the right assay platforms helps the chemistry team understand the mechanism of action of the PROTAC compounds.
Syngene is currently running several integrated drug discovery research programs with multiple targets to screen and identify molecules that are superior in terms of solubility, cellular permeability, and degradation capabilities. Currently, we are engaged in expanding our existing biophysical, biochemical, and cellular assay toolbox by introducing new technologies or new assays to provide new insights into the mode of action of PROTAC degraders.
Assessment of Druggability
Optimized degrader molecules have fast rates of degradation and relatively short exposure to therapeutic doses that can result in the complete elimination of the target protein. This helps in obtaining a durable and sustained effect, subject to protein turnover rate. Our optimized technology and assay platform can be utilized for any soluble target and bring discovery efforts on a fast track for research programs of interest in multiple disease areas. Syngene has the capability to optimize a number of properties for any degrader to enhance its druggability. This includes its solubility, permeability, ability to bind tightly to proteins, ensure efficient functioning in vivo, and thermodynamic stability. We use a battery of assays to analyze these properties before pronouncing a new heterobifunctional degrader ready for biological use. These include:
In vitro ADME assays
Solubility (kinetic)
LogD
Plasma and whole blood stability
Metabolic stability in hepatocytes (mouse – most preferred, rat, rabbit, dog, monkey, and human)
Bidirectional Caco-2 permeability – specially designed for PROTACs
UC-PPB (custom-based with stability assessment) and RED
Cocktail CYP inhibition
Time-dependent CYP inhibition (need-based)
Metabolite identification (value-added services) in hepatocytes – specific to PROTACs
Preclinical formulation development and stability studies
PK studies
Cassette PK studies in mice
Single-dose oral and IV PK studies
Tissue exposure studies
Blood and plasma PK studies
PK-PD studies are challenging for PROTACs for the reasons described above and to establish a correlation. Syngene team is using different approaches for drawing the PK-PD correlation followed by testing the molecule in the animal efficacy models based on disease relevance.
Syngene’s capability in developing degraders
The degrader toolbox is growing rapidly, with many pharmaceutical companies investing in this revolutionary modality, especially for diseases like cancer. By partnering with CROs/CDMOs like Syngene, who have expertise in TPD and a deep understanding of the ubiquitin system, biotechs can scale operations quickly and cost-effectively. Syngene has significantly optimized the various steps involved in taking a degrader from bench to clinical development, thereby cutting down the time and effort needed to identify the right TDP approaches successfully.
While inhibitors need to achieve a high degree of target engagement over an extended period of time to exert their sustained pharmacodynamic effects, PROTACs act catalytically and are ready to bind to another molecule of a target protein after a limited engagement with it.
This mechanism, along with target specificity, can help in improving the therapeutic index and minimizing safety liabilities. Syngene’s drug discovery efforts which involve step-by-step profiling of compounds in sync with platforms associated with Ubiquitin proteasome system and pathway, help medicinal chemists design better molecules with physiologically relevant effects. In a nutshell, we are a one-stop solution for all TPD research requirements across various molecules, including heterobifunctional degraders and molecular glues.
About the authors









