Introduction
A poor technology transfer has many ramifications – problems with initial batches, analytical testing, packaging, and potentially, patient safety. Conversely, a successful technology transfer ensures that product quality does not suffer, while also saving time and resources.
This Point of View explains how Syngene, a leading global CRO/CDMO, has devised a systemic approach to achieving ‘100% first time right’ technology transfer in Biologics manufacturing. The methodology comprises a risk-based approach for assessing incoming data from the client-side. This involves assessing confidence levels for processes and analytical methods using a Decision Tree, arriving at risk mitigation strategies, and finally executing the transfer using a detailed Technology Transfer approach document to achieve ‘100% first time right’.
The methodology has resulted in a track record of consistent yield, regardless of differences in infrastructure, equipment, raw materials, processes, and more, between the client’s site and Syngene.
The transfer of technology is an integral part of a product life cycle. The international conference on harmonization (ICH) Q10 guidance advocates technology transfer to be executed under good manufacturing practice (GMP) – given its relevance to commercial manufacturing and impact on product quality. This emphasis is justified due to the complexities surrounding the transfer of technology.1 The 4Ps of technology transfer can be grouped into hard (prototype and patent) and soft components (process and people). These four elements form the basis of good practices in technology transfer.
Goals and objectives of Technology Transfer
The goal of technology transfer is to systematically transfer product and process knowledge within or between manufacturing sites to achieve product realization. This knowledge forms the basis of the manufacturing process, control strategy process, validation approach, and continual improvement.2 The objectives of Technology Transfer are as follows:
- Explain the processing information to enable technology transfer from R&D to the production site
- Explain the processing information to enable technology transfer of an already existing drug substance from one production site to another production site
- Illustrate specific procedures and points to be considered for the above two types of technology transfers to ensure a smooth transfer of technology.3
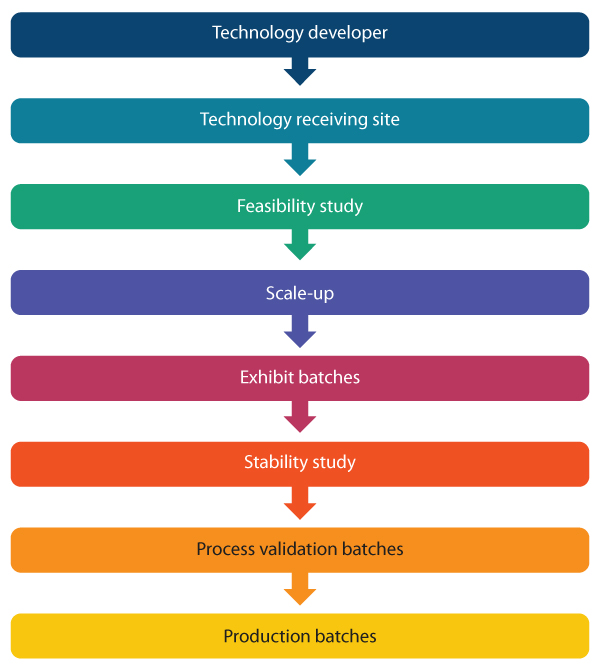
Challenges in Technology Transfer
The main challenge in technology transfer for contract development and manufacturing organizations (CDMO) and clients is the timely completion of milestones within approved budgets. However, working effectively with limited small-scale data is more critical for a CDMO. This is because it directly impacts the project cost and timelines, thereby influencing the relationship with clients. Regardless of the motivation for the technology transfer, the main goal of the receiving site is to execute the manufacturing process with little or no changes to the sending site’s original process. The task becomes even more difficult in the case of the following scenarios:
• The process developed initially is suitable for small-scale production in a laboratory but inappropriate or difficult for scale-up • The process requires certain unit operations or raw materials that do not comply with GMP
- There are differences in the type and capabilities of equipment available between the sending and receiving sites
- Poor documentation of the existing process and ambiguous description of the final process to be transferred
- Lack of clear and open communication between the sending and receiving sites.
- Aggressive timelines because of time-to-clinic or time-to-market pressure combined with a limited budget
Syngene’s track record in Technology Transfer
Syngene has a good track record of data integrity, data security, and protecting the intellectual property (IP) of its customers (a critical aspect of technology transfer). Over our 27-year history, we have built deep, trust-based relationships with our clients as an innovative, reliable, endto-end solutions provider. Our track record in technology transfer is as follows:
- Successful transfer from client laboratory to Syngene manufacturing site
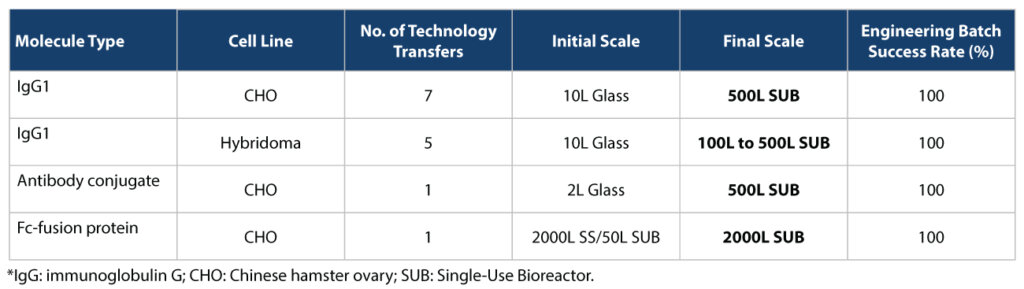
- Process performance, in-process attributes, and final product quality, always meeting client expectations
- No failure of batches – systematic approach and due diligence leading to ‘first-time right’ transfer
- Scientific and practical knowledge helping to manage technical challenges arising from equipment or infrastructure differences and ultimately resulting in smooth scale-up
Our integrated strategy for Technology Transfer
As shown in Figure 4, Syngene uses an integrated strategy for technology transfer. This consists of a Development group (left side of the figure) and the GMP group (right side of the figure). In addition, Syngene also has several integrated models between Development and Manufacturing to ensure a smooth transition between the two. We also undertake activities like stability studies, method validation, and process characterization as part of the technology transfer process.
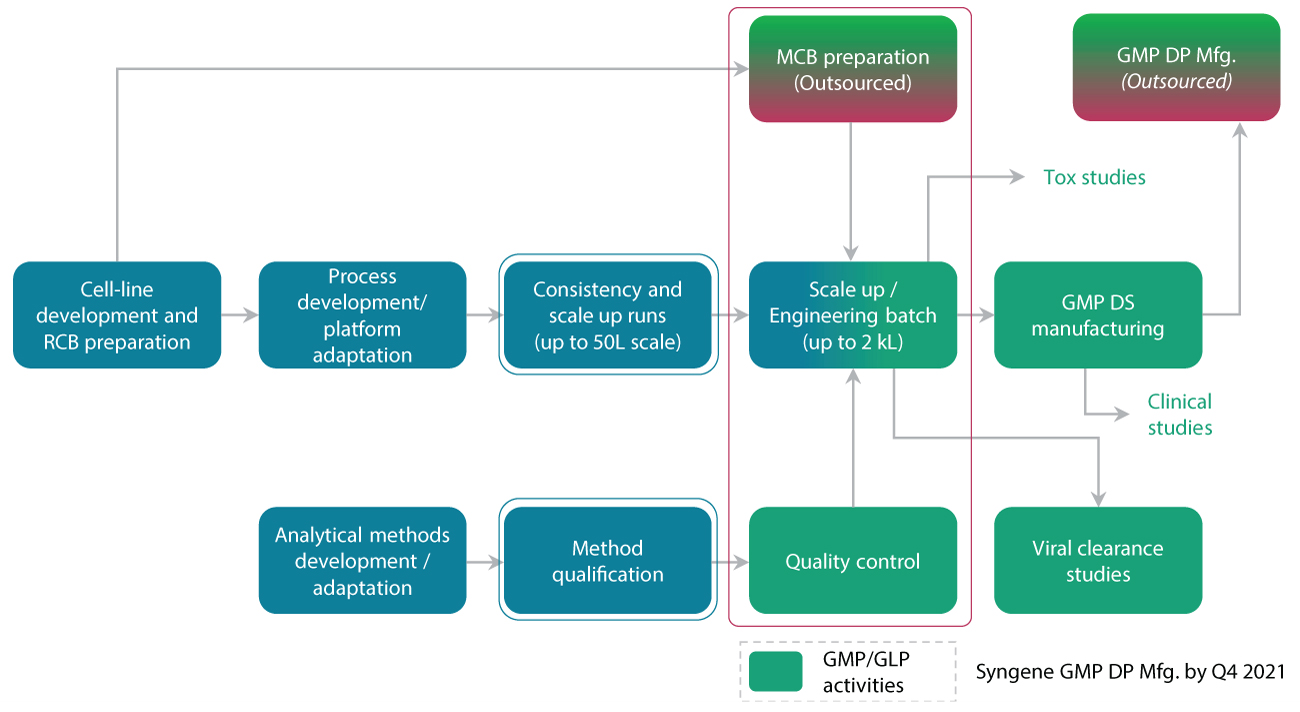
Syngene’s Technology Transfer approach
Syngene collects essential information from clients to ensure ‘first-time right’ transfer. This comprises information about processes and analytical methods, the kind of equipment, raw material, and consumables used during development, and the final product quality targets and specifications. Syngene has developed a risk-based systemic approach for assessing the incoming data (process and analytical) based on the requirements of the project.
These assessments form the basis of the technology strategy to be used depending on the confidence level (high, medium, and low) emerging from this analysis (refer figure 5)
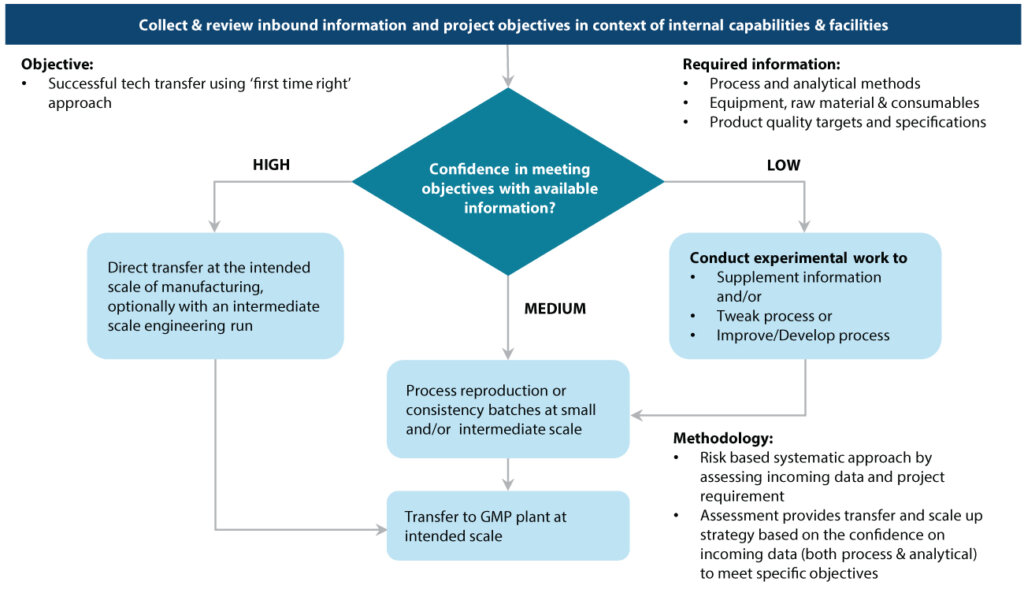
Assessing confidence levels on processes using Decision Tree
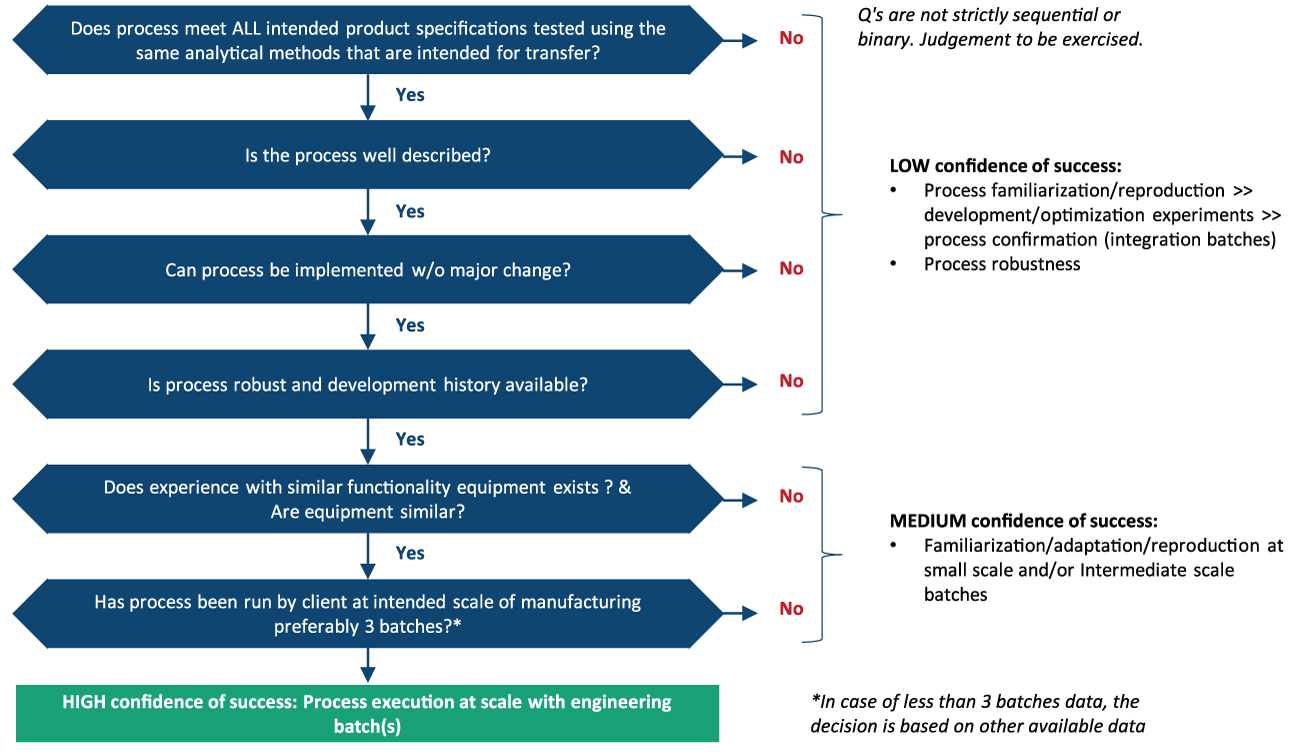
Syngene ensures that it is absolutely candid about its understanding of the client systems and controls to enable a successful technology transfer. The Decision Tree approach helps us assess our understanding and confidence levels in undertaking the transfer. The Decision Tree approach comprises multiple sections with multiple questions and sub-questions under each section. The questions are not sequential and have been established internally by Syngene to assess desired outcomes. The first set of questions involve questions related to product specifications.
The second section comprises questions related to the processes involved. The third comprises questions to assess the risks involved and to figure out if any changes are required to the processes to avoid the risks. The fourth focuses on understanding the data available based on the product development history and process. Syngene’s Decision Tree process of assessing confidence levels using sequential absorption of technology is depicted in figure 6.
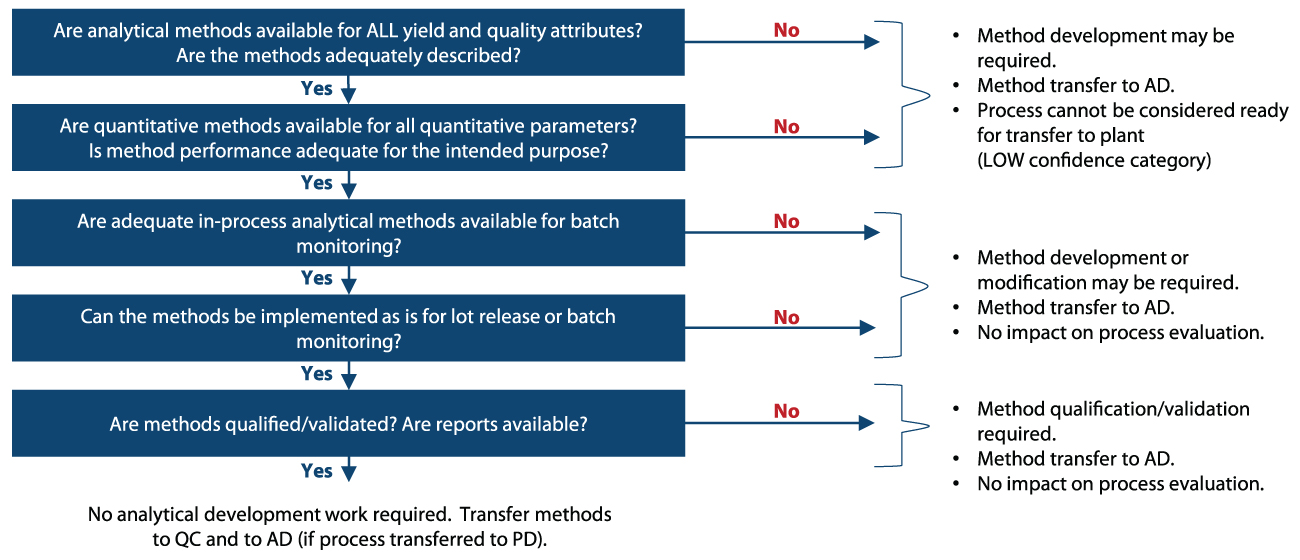
Assessing confidence level on analytical methods using Decision Tree
Just as with client processes, we apply the same Decision Tree approach to assess confidence levels in analytical methods used by the client before undertaking the transfer. Having all the analytical information in place is critical to ensuring successful technology absorption. Questions are posed to clearly understand whether a new method needs to be developed or not, or if analytical method modification is enough to match the equipment at the client’s end.
If there are information gaps, the confidence level is categorized as low . If a slight modification of methods or adaptation of X equipment to Y is required, the confidence level is categorized as being in the medium category, and we move on to the next stage. In cases where the processes are a perfect fit, the technology is transferred to our site with simple qualification and validation. This falls under the high confidence category.
Next comes validation and qualification of processes. To do this, we first list out the methods that can be sent directly to the quality control laboratory. Next, we validate the methods in-house before using them for GMP batches. We use qualified methods for our engineering batches. This is a prerequisite for process validation or product quality analysis at Syngene. Further, we use GMP-validated procedures for GMP batches relating to all practices and systems. The Decision Tree helps us achieve our manufacturing milestones – lab to engineering to GMP scale. Our analytical methods using the Decision Tree process are represented in Figure 7.
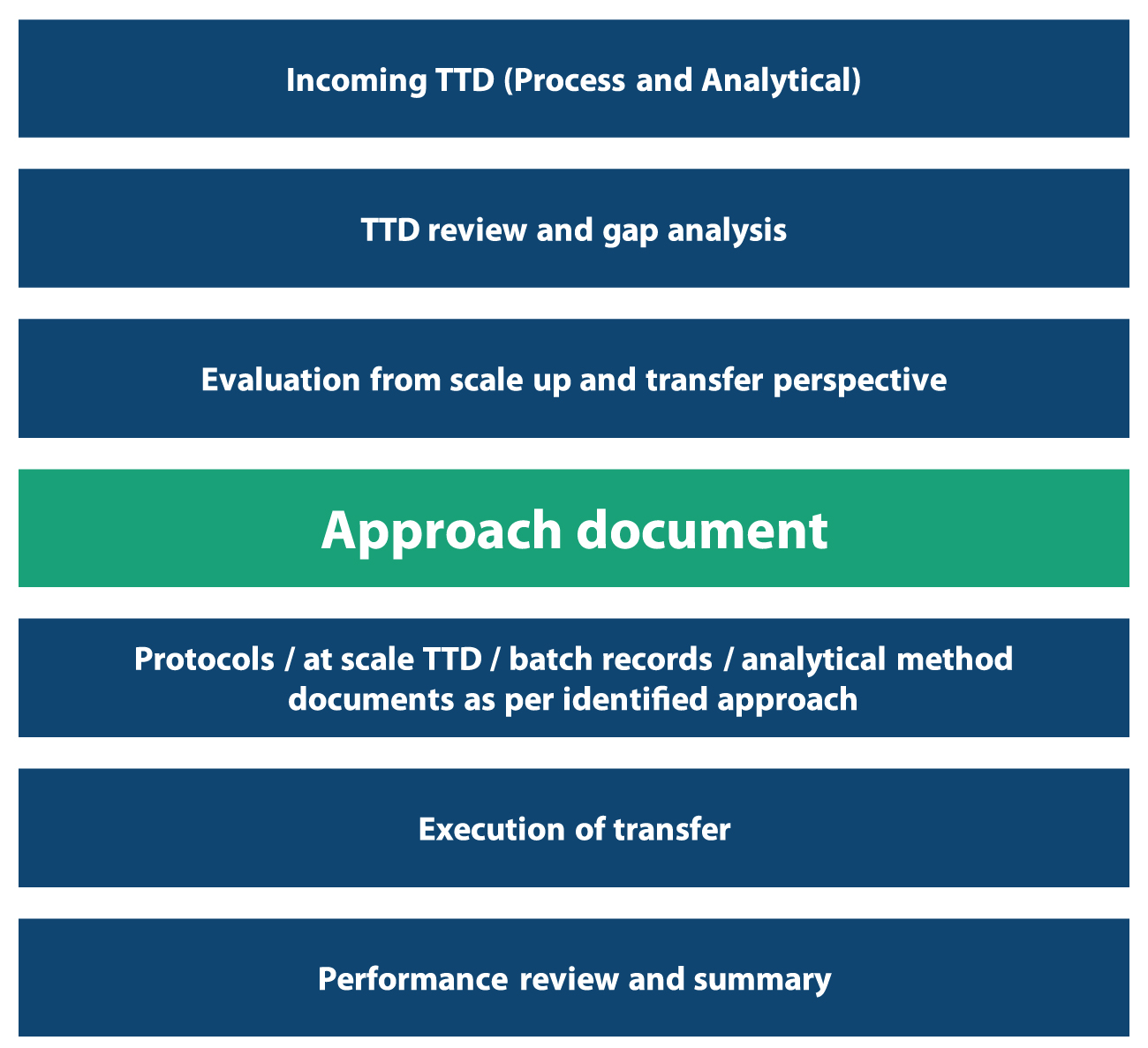
Preparing the Technology Transfer approach document
An approach document describes the gaps in the technology and facility between the sending and receiving sites, compares process parameters and equipment between both, identifies risk, and provides appropriate mitigation strategies. At Syngene, we have our own Technology Transfer approach document with a list of questions to capture as much information as possible about the drug substance from the client.
We also accept any development data the client may have, which could affect scale-up operations. Once we have received all the data, we analyze it to measure our confidence levels in replicating these processes and analytical methods for manufacturing. In case any of the parameters are not well established or understood, we check it out ourselves in our own development laboratory. We then set up the parameters and establish the processes at our site required for scale-up operations. However, this would add more time to achieving the final output. Once all the data has been collected and protocols defined, we derive the final approach document complete with risk mitigation strategies in place to execute the transfer. The technology transfer document (TTD) process flow is represented in figure 8.
Upstream and downstream scale-up strategies
Syngene has a state-of-the-art Biologics manufacturing facility for upstream processing of multi-product cell culture. Operations are carried out on a single-use platform catering to clinical and commercial manufacturing requirements. The facility offers two suites (from inoculum to production) having a production capability of multiple 2000-L, Single-Use Bioreactors (SUB). In most cases, Syngene uses the KLA (target performance metric) approach, where a process is transferred from one bioreactor platform to another. We also take into account, power input required for production on a broader scale. We ensure the shear stress is under control, and we have an appropriate mix of testing strategies to ensure a successful technology transfer from the client-side to our side.
As part of the downstream process during normal conditions of chromatography, we keep a constant bed height for the non-linear scale of the processes. In addition, we ensure that the residence time is maintained. As part of the filtration strategy, we ensure the parameters of the protein rate per unit area or flow rate are maintained. We also make use of hold-time data to determine how quickly we can assimilate the technology. This is important because if there is no awareness about hold time, it could lead to compromising on quality during processing.
Critical upstream scale-up parameters, bioreactor parameters, and bioreactor characterization
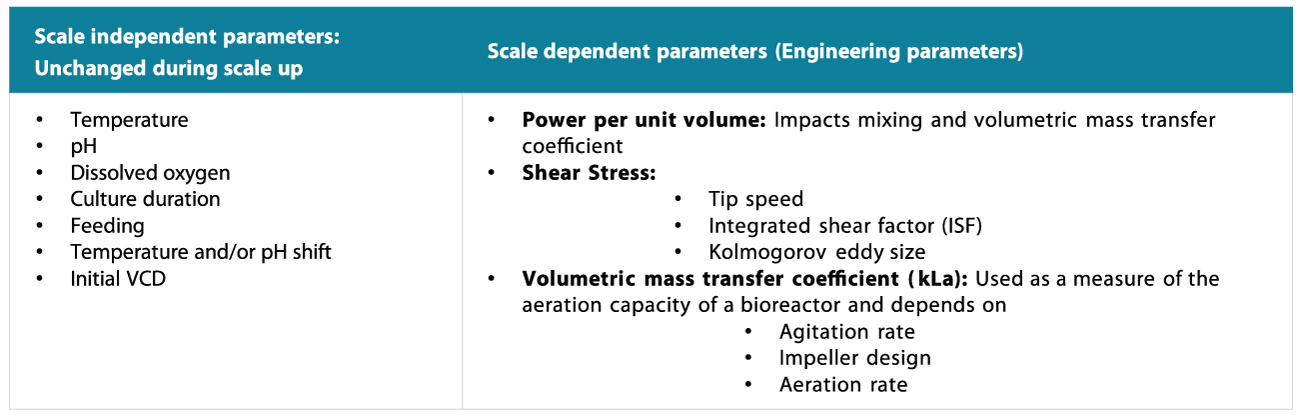
Critical upstream parameters are generally categorized as independent parameters that do not change. On the other hand, scale-up parameters depend on what the client wants us to focus on, i.e., shear stress and volume mass transfer are part of the criteria for the power input for volume (P by V), Syngene ensures that the scale-dependent/independent parameters are transferred with a focus on the scale-dependent parameters rather than on the independent ones.
To enable this, we have well-characterized bioreactors across different scales. Bioreactors are thoroughly studied at Syngene to derive scale-dependent parameters. Figure 9 represents some dependent and independent scale-up parameters for bioreactors.
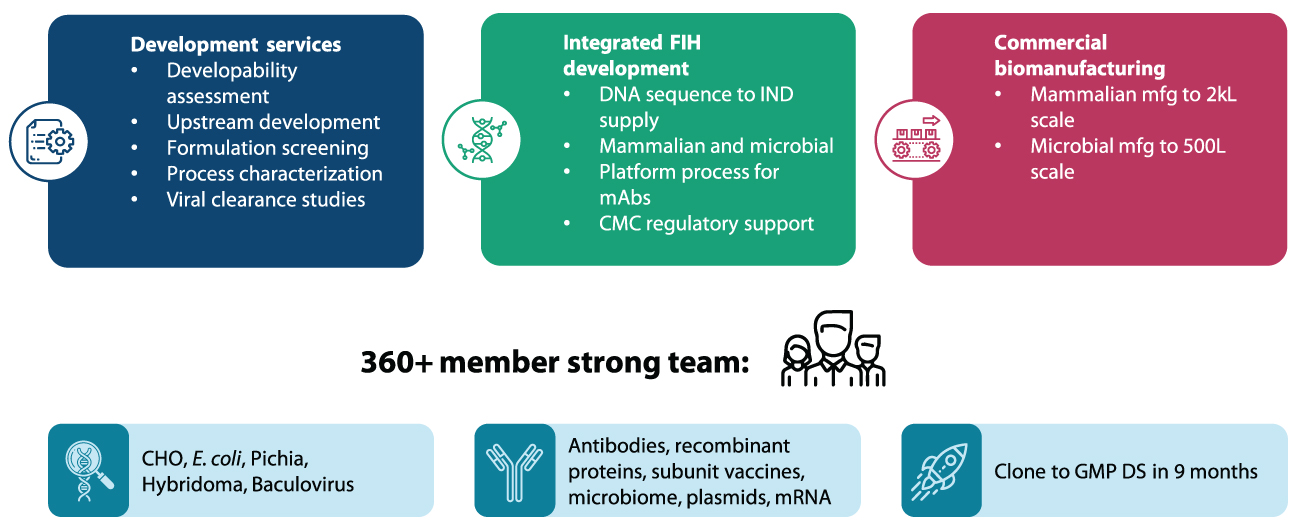
Syngene’s capabilities in Biologics
Syngene’s Biologics division is divided into three primary units – Development services, Integrated First-in-Human (FIH) development, and Commercial manufacturing. Once a product has passed through various development processes like developability assessment, upstream development, formulation screening, process characterization, and viral clearance studies, it is integrated with processes in FIH development. These include DNA sequence to IND supply, platform processes for mAbs, and finally, CMC regulatory support. This is followed by commercial manufacturing.
Syngene can undertake commercial manufacturing up to 2 kL on the mammalian side and 500 L to scale on the microbial side. All the services are delivered by a 360+ strong scientific team with a cumulative leadership experience of 20+ years and execution experience of 10+ years.
Conclusion
The success of any technology transfer is contingent on a thorough grasp of the process or the capacity to predict its future performance accurately. The plan, the process, and the people involved are the three main components in a successful technology transfer. Syngene has all these attributes, including developmental capabilities, integrated modules, and state-of-the-art facilities, making us a one-stop solution partner for Technology Transfer. Over the years, we have successfully executed several technology transfer projects in Biologics while ensuring consistent yield. Our process performance, in-process attributes, analytical methods, and final product quality have always met client expectations. We have absolutely no history of batch failures as a result of our systemic approach and diligence in ensuring ‘first-time ‘right’ technology transfer. Further, our scientific and practical knowledge helps us manage technical challenges regardless of differences in infrastructure, equipment, raw materials, consumables, and more.
In a nutshell, our unique approach to technology transfer provides the following benefits to clients:
- Timely achievement of clinical and commercial milestones
- Compressed time-to-market expectations
- Speeding up last-mile delivery (products to patients)
- Reduced Costs
References
1. Pharmaceutical Quality Systems Q10, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2008.
2. Manral MS, Prashar B, Sheikh Y. Technology transfer in the pharmaceutical industry; facts and steps involved. Am J Pharm Tech Res. 2012;2(4):74-82.
3. Sagar P, Akshay K, Pankaj P, Mayur G, Shivram J. Review article on technology transfer. Int J Pure Appl Biosci. 2014;2(3):145-153.
About the author








