Introduction
Biopharma Outsourcing has come a long way in recent times. From assigning only commoditized work to service providers, it has progressed to partnering with them. Reasons include access to specific technologies, skills, and scientific expertise not possessed in-house. As a result, the criteria for choosing an outsourcing partner (contract research or contract development and manufacturing organizations [CRO/CDMO]) have also changed.
In this point of view, we discuss the criteria that have emerged as more important for selecting an outsourcing partner consequent to disruptions caused by the COVID-19 pandemic. The criteria are based on customer feedback and recent meetings with prospects, peers, and partners during on-ground biopharma events across the US, Europe, Australia, Japan, and the rest of the world.
Revised criteria for choosing CROs/CDMOs
Supply chain resilience
The COVID-19 effect was felt deeply in the biopharma industry, as national lockdowns and border closures brought into question the practice of using long and highly globalized supply routes, including overreliance on China. Companies had set up these routes to cut costs and diversify their business in an era of seamless cross-border trade. When the pandemic struck, it led to a supply crunch for key raw materials. This, in turn, resulted in delays/disruptions to projects in the discovery/preclinical stage, including affecting c linical a nd commercial s upplies. Collectively, it impacted the progress of science, leading to deferred clinical trials and limited access to new treatments and therapies for patients worldwide.
Biopharma companies have since realized the increased risk of such strategies and are looking at ways to mitigate them. Their focus has now shifted from a vendor risk mitigation approach to a supply geography/ region risk mitigation approach. However, they also expect partners to have resilient supply chains, strong local supplier networks, and little or no reliance on a single country/ region.
Broad suite of capabilities and an integrated approach
The COVID-19 pandemic brought to the fore the advantage of having a single partner with a broad suite of capabilities across the drug discovery and development continuum as opposed to multiple specialty partners. It was observed that CROs/CDMOs with this capability could manage projects centrally, unhindered by logistical/shipping delays or dependencies on third-party deliverables. This enabled the timely completion of projects despite a raging pandemic. Additionally, it afforded customers the benefits of ease of management, efficiency, and cost savings.
The situation was in stark contrast to companies that had outsourced their requirements to multiple partners to gain cost efficiencies or avoid the risk of depending on a single provider. Customers were also seen to have shifted from a project-driven outsourcing approach to an integrated project approach.
This was to accelerate development timelines – given the escalating R&D costs and the need to cash in on the prime-mover advantage. An integrated technology platform would provide an effective and efficient means to conduct target validation, translational interrogation, therapeutic discovery, and preclinical development, including moving into the development phase. Such a platform would also allow teams to work collaboratively with customer counterpar ts to identify promising candidates and rapidly advance programs toward the target product profile in a virtual mode (in case of similar disruptions caused by COVID-19). Further, it would result in faster and more appropriate dossier preparation for IND and NDA filing resulting in a higher chance of success with regulatory authorities.
Manufacturing capacity and experience
Biopharma companies prefer CROs/CDMOs who can seamlessly scale manufacturing from lab-scale volumes to commercial levels. With this capability, they can outsource manufacturing requirements across clinical supplies, registration batches, and commercial supplies to a single service provider. This, in turn, would result in faster new drug application (NDA) submission and go-to-market opportunities for innovator companies.
Robust program governance
With travel restrictions hampering frequent supervisory visits by customers, there is an increased emphasis on strong project management skills at the partner’s end to ensure projects are on track. Integrated projects also call for higher program management skills to move seamlessly through drug discovery, development, and commercial manufacturing stages. Smaller biopharma companies who have much more riding on the success of their projects also expect professional management of their outsourced projects regardless of budget size or external circumstances. In brief, customers are looking for agile, collaborative, and seamlessly connected internal and external governance structures to operate efficiently.
They expect governance structures to be geared toward proactive risk management and quality and performance management. Yet another expectation is frequent and clear communication between internal and external governance teams from project initiation to delivery to ensure the timely delivery of projects with the desired quality.
Technology adoption
The last two years have shown the world the power of technology and the ability of humans to adapt technology to get the work done. This has resulted in customers expecting increased use of automation and digitization to deliver projects more efficiently. Customers also spoke about looking forward to more immersive interactions with their outsourcing partners in the not-so-distant future using technologies like metaverse, artificial intelligence, and augmented reality. They also expressed the need for real-time access to projects delivered by partners (often millions of miles away) to give them better control over outsourced projects.
How Syngene measures up to the above criteria
Supply chain resilience in biopharma
With an annual spend of Rs 20 billion, 77,000 purchase orders, 160,000 materials, and 2500+ suppliers across 30+ countries, Syngene’s supply chain is complex and robust, to say the least. In this, our 28 years of experience in serving global customers has been our guiding star. Our Supply Chain is powered by a supply ecosystem covering India, China, and the rest of the world (ROW) and a strong business continuity plan (BCP). The BCP encompasses risk review mechanisms, risk mitigation plans for sourcing and logistics, and advance material ordering and inventory planning aligned to specific drug discovery, development, and manufacturing requirements for small molecules and biologics. This strategy has helped us reduce procurement lead times, minimize supply failures, focus on quality, and influence business decisions.
Syngene’s Business Continuity Plan for supply chain operations
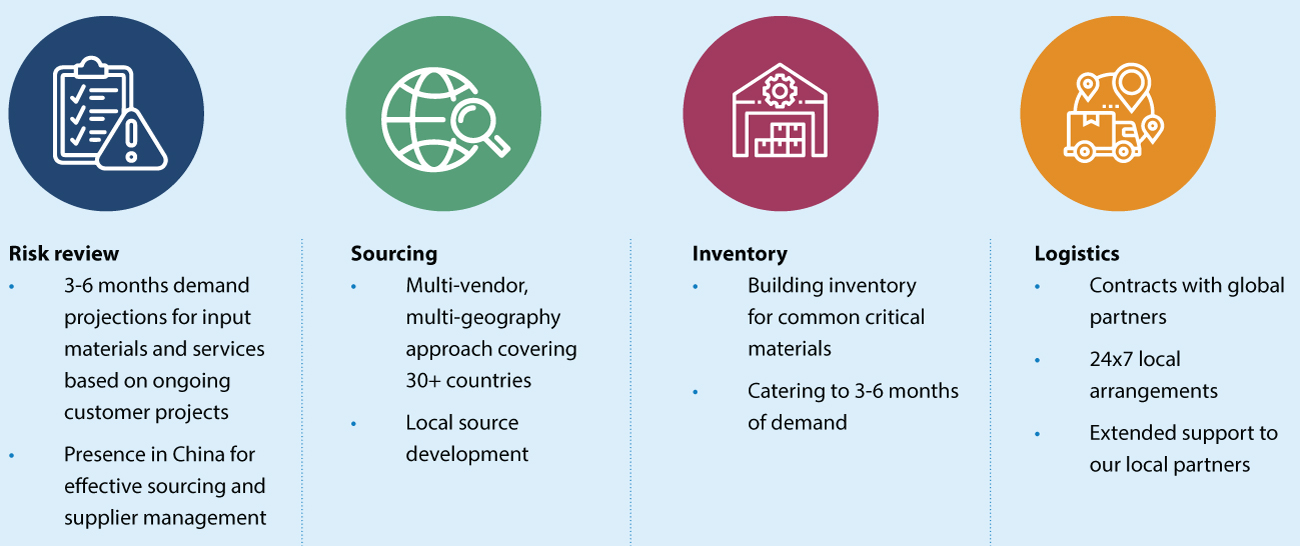
To further strengthen our supply chain, we have undertaken a major transformation of our supply chain system recently. Our procurement approach is no longer just fulfillment-based but more strategic in nature, with long-term benefits in view. The transformation program has been designed to build a world-class supply chain with a focus on the four pillars of People & Organization; Process; Governance & Sustainability; and Supply Ecosystem. While the focus on People & Organization revolves around building procurement teams with a skill-based partnering approach, the Process pillar initiatives involve automated/digitized processes with data-driven performance management systems to aid continuous improvement. In order to strengthen Governance mechanisms, we have developed a purchase manual, set up a purchase committee, enforced contractual penalties for non-compliance, and established a sustainable procurement policy that includes mandatory compliance requirements for antibribery/anti-corruption, etc.
Our supply ecosystem is designed to follow a differentiated strategy to meet the specific business challenges across drug discovery, development, and manufacturing for small molecules and biologics. For small molecule research businesses, faster delivery of catalog chemicals is our top priority. To cater to the requirements of our development business, we have built a strong network of custom synthesis suppliers in India to handle novel molecules that require relevant technical expertise in complex chemistry. For cGMP material requirements, supply chain integrity is a critical area of focus, as many of our customers are innovators.
To ensure cGMP supply chain integrity, we have robust processes in place across the entire supplier life cycle, from supplier selection, qualification, and performance management. For biologics, the non-availability of multiple sources for critical materials and the concentration of suppliers in the US and Europe are major hurdles for CROs and CDMOs. Reducing the lead times (stretching up to several months for some materials due to a global demand triggered by COVID-19) and making materials available for operations (supply security) are the key objectives of our supply chain function. While we have formulated our inventory strategy for long lead time materials, we have also started developing alternative sources in the domestic market/other geographies as a long-term solution. This approach has helped us deliver on our commitments to our customers – on time and with the desired quality even during the pandemic.
Comprehensive capabilities and integrated approach in drug development
Syngene is among the few CROs/CDMOs that can offer comprehensive services across the drug discovery and development continuum – all from one campus. This has helped us drive cost efficiencies and enable on-time delivery unhindered by logistical or supply chain issues. Further, Syngene’s SynVent platform enables fully integrated therapeutic discovery and development across large and small molecules. The platform can simultaneously apply multiple modalities for target validation and therapeutic discovery as per a specific target or pathway. With seamless integration into development stages also available, SynVent offers a hasslefree outsourcing experience that is outcomedriven and quality-focused. All this, while adhering to R&D timescales, benchmarked to industry gold standards.
cGMP manufacturing capabilities – small molecules and biologics
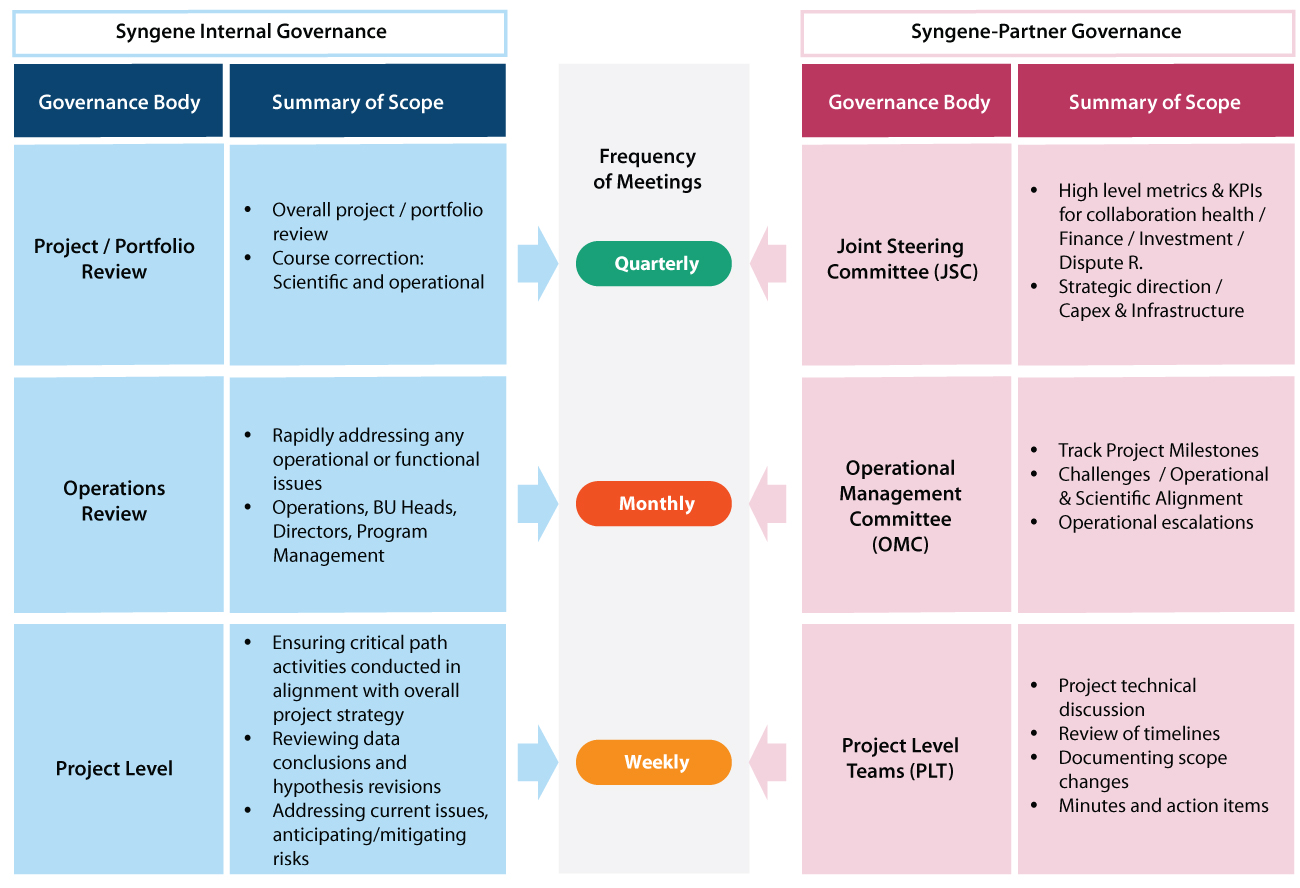
Using our current good manufacturing practices (cGMP), you can seamlessly scale from lab-scale volume to commercial levels for both small molecules and biologics requirements. We follow a phase-appropriate manufacturing philosophy in our facilities, which complies with regulatory guidelines. We have demonstrated capabilities and experience in manufacturing batches of compounds for preclinical, clinical, and commercial applications at appropriate facilities. For small molecules, we under take manufacturing for batch sizes between < 100 kgs to 400 kgs output/batch. For biologics, we offer GMP manufacturing capacity for mammalian (up to 2000L) using single-use bioreactors and up to 500L scale using stainless steel fermenters for microbial. Figure 2: Syngene’s Program Governance framework We have a Sterile Fill-Finish facility to manufacture ready-to-use solutions and lyophilized products (pre-sterilized vials and prefilled syringes) with occupational exposure limits ≥ 1 µg /m3 in a GMP environment. We are also the only GLP/ GMP Viral Testing facility in India for batch release and viral clearance testing – phase 1 to commercial.
Robust program governance framework
Testament to our program governance proficiency is that we currently manage projects related to 420+ global customers. We have 15 collaborations with the Top 20 pharmaceutical companies in the world. Our long-term partnerships include Baxter and BMS (Bristol Myers Squibb), which are over a decade old. Further, almost 79% of our revenues come from existing customers.
Technology adoption
In the past two years, Syngene adopted several measures to make the company more agile and responsive using technology. Some of the initiatives are given below.
Virtual inspection facility
At Syngene, even during a complete lockdown, we completed 33 facility inspections by regulatory authorities using information communication technologies (ICT). This facility helped us get regulatory approvals without anyone physically visiting our campus or facilities. Using these tools, regulators/customers can communicate, share, review, and access our systems without being physically present at the location where the activities subject to inspection are being carried out. We have also made available 30+ videos that provide a 360-degree view of our facilities – anytime and from anywhere. This unique platform allows you to change your perspective, look around, and zoom in/zoom out as you are taken on a guided tour of Syngene’s facilities and laboratories across Operating Units.
Remote Analytical labs
To augment our PROTAC capability, we set up high-tech Analytical labs in our Hyderabad campus, which have server-based software for all instruments, among other features. Scientists can use them to work in labs remotely and monitor progress using an e-dashboard. With this facility, scientists can now do critical research work from home with minimum visits to the campus for lab-related work – a critical requirement during a pandemic and similar situations in the future.
Digitization initiatives
We incorporated several digitization and automation initiatives in supply chain management to ensure increased operational agility. For example, our Strategic Sourcing team uses a Data-driven performance management system to drive continuous improvement measures across its teams. The system generates lagging and leading indicator reports which are periodically published to report performance and communicate the future outlook of sourcing activities to key stakeholders.
We also enabled system-to-system connectivity between Syngene and all our external supply chain partners – making the procurement process for raw materials seamless, fast, and efficient. Further, all our shipments now have Trace and Track features connected to Syngene systems, providing visibility into cargo movement at all times. Syngene plans to invest in many more cutting-edge tools/technologies in the coming years to be at the forefront of science and to ensure customer convenience from a governance standpoint.
Conclusion
The COVID-19 pandemic exposed the vulnerabilities in CRO/CDMO relationships as they existed pre-pandemic. The fallout of this experience was a revised set of criteria for evaluating partners for outsourcing. The changed criteria include having a future-proofed supply chain management system, a broad set of capabilities and an integrated approach to drug discovery, flexible manufacturing capacity, a robust program governance framework, and digitized/automated systems to drive faster and more efficient delivery.
Syngene stands tall on all these criteria and more. As a global CRO/CDMO with 28 years of scientific experience, 420+ active customers, and 400+ customer patents to its credit, Syngene is well-equipped to deliver on customer expectations and beyond. By partnering with us, biopharma companies can reap the benefits of reliability, cost efficiency, and speed in taking their molecules from early discovery to commercial in an accelerated timeframe – pandemic or otherwise.







