Introduction
Detection and quantitation of process-related impurities is an important part of quality control in biopharma manufacturing. Process-related impurities can significantly impact a pharmaceutical product’s quality, safety, and efficacy. These impurities may get generated during the manufacturing process, degradation, starting materials and reagents, by products, storage conditions, or contamination. If not properly detected and quantified, these impurities can lead to adverse effects on patients.
For this reason, ICH guidelines make quality by design (QbD) an essential requirement during pharmaceutical development. The ICH Q8, Q9, and Q10 outline a systematic approach to pharmaceutical development, emphasizing the critical importance of understanding product and process parameters to ensure product quality.
In this article, we discuss the challenges in the detection and quantitation of process-related impurities including nitrosamine impurities and Syngene’s capabilities and solutions to address them.
Challenges in the detection and quantification of process-related impurities
Several challenges can arise in the detection and quantification of process-related impurities using analytical techniques.
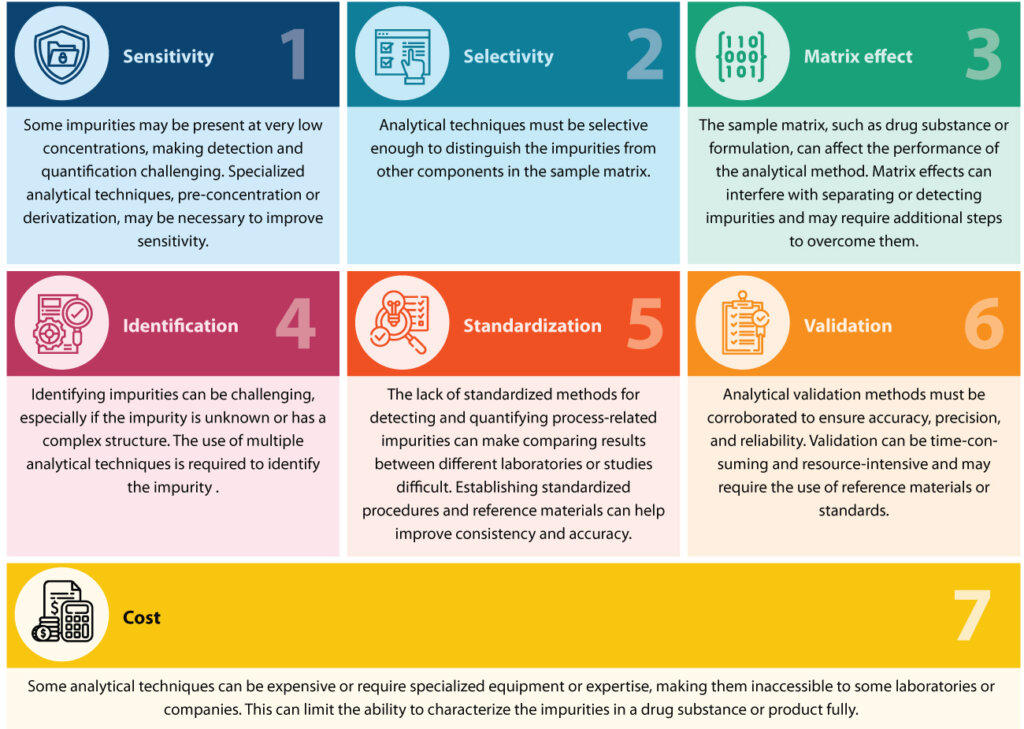
Syngene’s solutions for addressing process-related impurities
The detection and quantitation of process-related impurities are typically accomplished using high-end analytical techniques. These techniques
are used to separate, identify and quantify impurities in the drug substance.
Our services include providing the framework to identify the critical quality attributes (CQAs) to finalize the relevant process parameters during
product development. During this process, the emphasis is on using risk management tools such as failure-mode effect analysis and DoE to
identify and control potential sources of variability in the manufacturing process. This, in turn, facilitates the analytical justification of specifications.

Nitrosamine impurities
The recent and unexpected finding of nitrosamine impurities (probable human carcinogens in pharmaceuticals), such as angiotensin II receptor blockers (ARBs), ranitidine, nizatidine, and metformin, resulted in a large-scale recall of these drugs. Consequent to this finding, the FDA, in collaboration with regulatory counterparts worldwide, issued guidance to API and drug product manufacturers. The guidance was on appropriate actions they need to take to detect and prevent unacceptable levels of nitrosamine impurities in pharmaceutical products (Source: Control of Nitrosamine Impurities in Human Drugs: Guidance for Industry).
Syngene’s Center of Excellence for nitrosamine impurity testing
Syngene offers a state-of-the-art facility for risk assessment, development, and validation of nitrosamine impurities in drug substances and drug products. The facility supports Nitrosamine testing in drug substances in line with formulation requirements for small molecule APIs, key sourcing materials (KSM), and intermediates. Its internationally accredited Analytical labs and highly skilled scientists ensure all the data generated complies with regulatory requirements.
Key Features
- Diverse experience in nitrosamine risk assessment, method development and validation, and testing of nitrosamine impurities in drug substances, drug products, key starting material, and intermediates
- All methods developed and validated according to USP General Chapter <1469> in line with current scientific and regulatory approaches. This ensures appropriate control over nitrosamine impurities in APIs and drug formulations
- Availability of skilled workforce, including those with expertise in nitrosamine impurity testing
- Dedicated area for nitrosamine and azido impurity testing; nitrosamine and azido impurity characterization
- Availability of sophisticated nitrosamine analysis instruments such as LC-MS/MS, GC-MS/MS, and HRMS to quantify impurities at the ppb level as per regulatory requirements
- All nitrosamine testing conducted in cGMP labs audited by multiple regulatory agencies
Conclusion
Syngene has the expertise and capabilities, including state-of-the-art instrumentation, to provide comprehensive solutions for the detection and quantitation of process-related impurities in pharma and biopharma products. We also offer a state-of-the-art facility for risk assessment, development, and validation of nitrosamine impurities in drug substances and products. Our highly sensitive and selective methods allow for accurate identification and quantitation of impurities, even at low levels. The use of appropriate standards, controls, and validation procedures ensures the accuracy and reliability of our analytical results as per regulatory guidelines. This ensures the successful development and manufacturing of safe and effective pharmaceutical and biopharmaceutical products at all times.
About the author








