DMPK in Drug Discovery Overview
Accurate data and insightful analysis of the Drug Metabolism, Pharmacokinetics (DMPK), Absorption, Distribution, Metabolism, and Excretion (ADME) properties of your compounds is a critical part of the drug discovery journey and helps you shortlist candidates with the highest chances of success in the later stages of drug development. The right partner for your DMPK ADME needs can help you reduce compound failure, identify compounds with optimal safety profiles, and minimize drug-drug interactions in later stages.
In this point of view, we discuss some DMPK strategies to avoid drug development mistakes early. These strategies have helped Syngene scientists drive cost-effective, reproducible, high-quality data for our clients, thereby enabling faster movement of molecules from bench to bedside. So far, Syngene has worked with 117 global clients, conducting nearly 3500 PK studies/year.
DMPK Strategies
Studies indicate that though drug attrition due to PK/bioavailability has decreased over two decades, it still needs to improve significantly. Further, other DMPK parameters like efficacy, toxicology, formulation, and cost of goods continue to be underestimated as leading causes of drug failure. At Syngene, we have put together some strategies to overcome these hurdles and achieve our clients’ “druggability” wish list.
The wish list is as follows:
Oral administration and easy absorption
Has good permeability and aqueous solubility (Class I compounds)
Metabolically stable compound but not too stable
Needs to be taken only once a day
Has predictable metabolism
Linear metabolism kinetics
Allows balanced clearance
Secreted via renal or biliary secretions
Produces a limited number of inactive products
Metabolized by several P450s
Not dependent on polymorphic P450s
Does not inhibit or induce absorption, distribution, metabolism, and excretion (ADME) of enzymes
P450, UGT, and MDR1 (P-gp)
Has a small first-pass effect
Has a comprehensive therapeutic index
Key DMPK challenges in achieving the “druggability” wish list
How to avoid PK-based drug-drug interactions (DDIs)
In general, drug metabolites are more polar and can also induce or inhibit enzymes, especially hepatic enzymes, resulting in drug-drug interactions (DDIs) and drug-food interactions.How to optimize oral drug absorption
The key challenge to overcome here is to reduce the time required for a drug to move from the administration site to the blood.How to avoid central nervous system (CNS) exposure
Some drug discovery approaches can benefit from restricting the access of compounds to the CNS to minimize the risk of side effects.
Also, designing compounds that act as substrates for efflux transporters in the blood-brain barrier can help achieve CNS restriction without significantly impairing absorption in the intestine.How to optimize clearance
An ideal time of clearance needs to be standardized for each drug, depending on its mode of action, its intended purpose, its target tissue, and its toxicity profile.Accurately understand the role of biotransformation in drug discovery
Drug biotransformation reactions include oxidation, hydrolysis, and conjugation. A single drug may be transformed into several metabolites. One needs to keep in mind the effect of both genetic and external factors on the metabolism of the drug in different individuals.
Syngene’s strategy to overcome DMPK challenges
Syngene’s fundamental strategy for overcoming DMPK challenges and moving your candidate to the clinical phase is a two-pronged approach:
Modifying physicochemical properties: This is the simplest starting point for DMPK property optimization with quantitative structure-property relationship (QSPR) and aids efficient compound design
Improving the predictive capabilities of in vitro data: This is a key component for deriving an understanding of what is likely to occur in patients from both an efficacy and safety perspective
Although there are limitations to the utility of preclinical data, our risk mitigation strategy is built on an understanding of compound behavior in preclinical species. The goal is to extrapolate that understanding to predict clinical outcomes.
Over the years, our DMPK techniques have evolved, resulting in increased accuracy in predicting the safety, efficacy, and toxicity of compounds. In-silico predictions are currently the most preferred mode among clients compared to other techniques.
DMPK techniques and Capabilities at Syngene
- In-silico predictions for compound design
High-throughput assays
Rat hepatocyte Clint, human microsome Clint, aqueous solubility (pH 7.4), plasma protein binding, Log D, cytochrome (CYP) liability screening (3A, 2D, and 2C9)
High use of project-specific assays
Human hepatocyte Clint, CaCO-2, rat PK assays
Low use of project-specific assays
Dog PK and reactive metabolite assessments
Profiling assays
CYP DDI, transporter substrate and DDI, TDI, CYP induction, and rat/dog clearance assays
Problem-solving assays
Madin Darby canine kidney (MDCK) – MDR1 cells, Using chamber, rat biliary clearance, microdialysis, rodent receptor occupancy assays, and biotransformation assays
Key DMPK Optimization Strategies at Syngene
Optimization of oral drug absorption
Once a compound series is determined to have Caco-2 permeability<1×10-6 cm/s, we take appropriate steps to determine whether paracellular or active efflux plays a significant role and then eliminate this potential liability through structural modifications. We address potential risks and issues in oral absorption and bioavailability using a series of assays/techniques. These include intestinal microsomes or S9 fraction, PAMPA, GI stability test, in situ/in vivo portal vein cannulation preparation, etc.Avoiding PK-based DDIs
The four most common forms of clinical PK-based DDIs of which your compound may be the perpetrator or victim are:
-
Competitive (reversible) CYP inhibition
-
Mechanism-based/time-dependent CYP inhibition
-
Uptake and efflux transporter inhibition
-
CYP induction
DDI usually occurs with CYP, notably CYP3A4, in the liver or intestine. Transporter-based DDI is mainly related to renal clearance. However, specific issues could arise with CNS compounds, hepatic uptake of statins, and with drug absorption.
The potential for a compound to be a victim of a DDI with a co-medication is greatly reduced if there are multiple clearance mechanisms. This is especially true for those involving metabolism by multiple CYP enzymes. We use the following methods to phenotype the individual CYP enzymes responsible for a drug’s metabolism:
-
Specific chemicals or antibodies as specific enzyme inhibitors
-
Individual human recombinant CYPs
-
A bank of human liver microsomes characterized for CYP activity prepared from individual donor livers
Induction of specific CYP enzymes may change a drug’s metabolic profile and exposure and lead to toxicological consequences. This is because CYP enzymes are also involved in the metabolism and synthesis of important endogenous compounds.
Avoiding CNS exposure
We make every effort to keep the drug’s brain-plasma (B–P) ratios within the desirable range. Brain-plasma ratios can be deceptive—high B–P ratios may be a consequence of high nonspecific binding to brain tissue.
We use bidirectional transport assay, protein, brain-binding assay, and cerebrospinal fluid microdialysis to ascertain CNS exposure.
Optimization of clearance
This is one of the major challenges because clearance must be suitably low to achieve an appropriate half-life and bioavailability so that it can be used in humans.
Biotransformation Challenges and Mitigation Strategies in Drug Discovery
The three major areas of drug discovery that are dependent on metabolite identification and understanding metabolite properties are:
Assessment of pharmacologically active metabolites leading to PK–PD disconnect
Planning the safety assessment program leading to off-target activity
Avoiding/assessing risk from reactive metabolites leading to genotoxic or mutagenic issues
Our strategies to minimize or manage biotransformation risks are based on the following:
Metabolite identification
Trapping of electrophilic intermediates
Time-dependent inhibition
Assessment of acyl glucuronide stability
To address this challenge, we first identify the key elimination processes that determine clearance. We then optimize these processes through the use of human in vitro systems supported by animal data (i.e., in vitro–in vivo extrapolation). High metabolic clearance can be associated with many metabolic pathways:
CYP-mediated [Reduced nicotinamide adenine dinucleotide phosphate (NADPH) dependent] oxidation in the liver, and to a lesser extent, in other organs, such as the small intestine. Flavin-containing monooxygenases and monoamine oxidases can also be involved in oxidative metabolism
Direct or phase II conjugation through uridine diphosphate-glucuronosyltransferase, sulfotransferases, or glutathione S-transferases
Whole blood and tissue amidases, esterases, various amine oxidases (e.g., diamine oxidase and semicarbazide-sensitive amine oxidase), adenosine deaminase, AO and alcohol and aldehyde dehydrogenase may also come into play in some cases, depending on the chemotype
Failure to target appropriate metabolic clearance is a major issue in discovery because it could result in very high total body clearance, low oral bioavailability, or a short half-life. In turn, this may result in an inability to deliver an oral therapeutic drug and long-drawn discovery timelines, ultimately resulting in the death of a project because an alternative chemotype could not be found.
To reduce CYP-related clearance, we undertake the reduction of electron density or removal of metabolically labile functional groups. Some other strategies include understanding metabolic soft spots and their effect on clearance [MetaSite™] and orientation to the active site of CYP, and affinity modification of the substrate to the enzyme.
What Sets Syngene Apart in DMPK Services for Drug Discovery
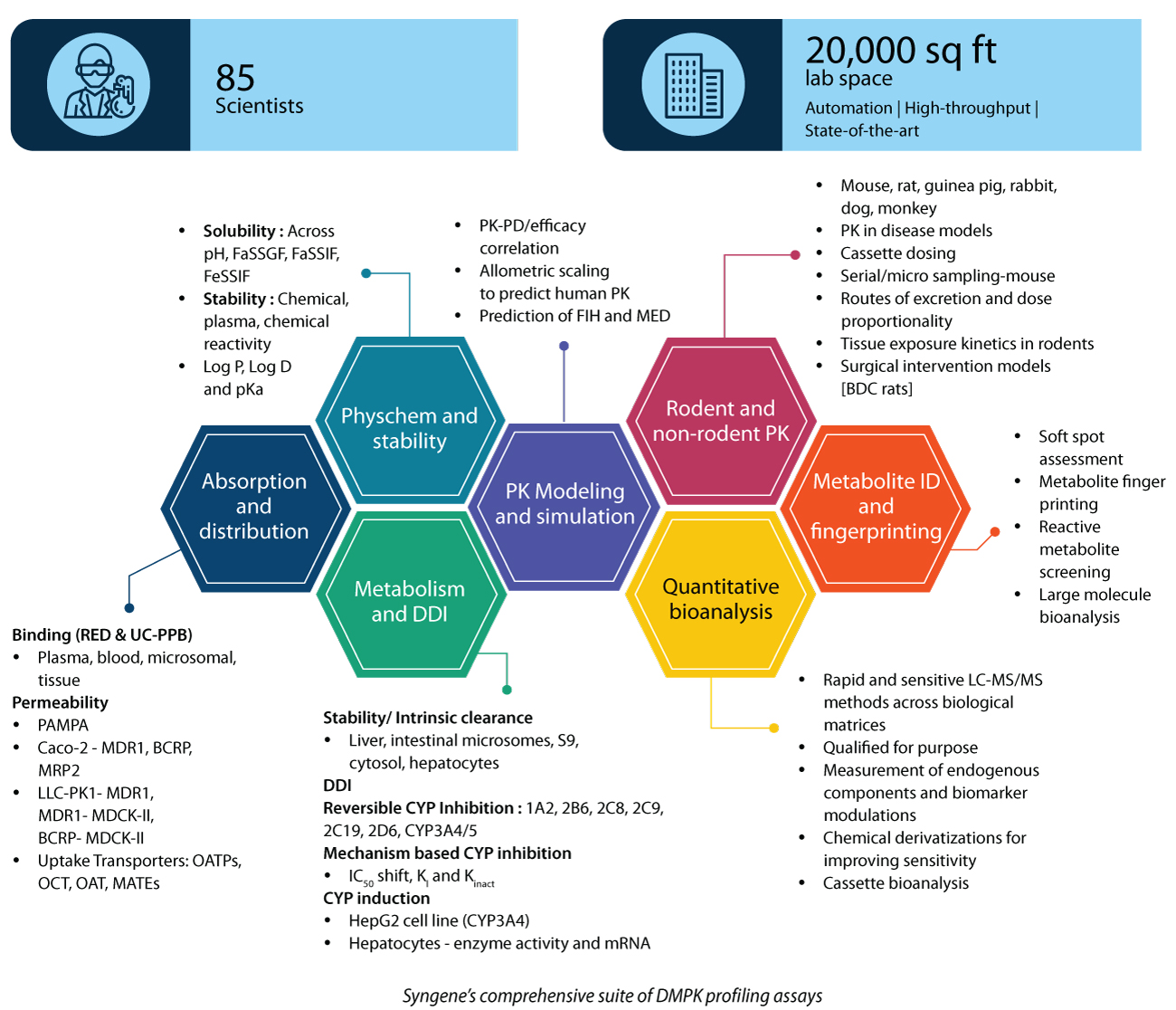
Syngene, with its comprehensive suite of DMPK profiling assays, can help you achieve a high rate of success with your clinical candidates across drug modalities. So far, Syngene has worked with 117 global clients, conducting nearly 3500 PK studies/year.
Here are the top six reasons why clients choose Syngene for DMPK services:
Full suite of DMPK studies: In silico, in vitro ADME, in vivo PK (rodent and nonrodent), and metabolite ID studies across discovery and early development programs
Services across modalities: Small molecules, proteolysis targeting chimeras (PROTACs), peptides, antibody-drug conjugates, and oligos—HT screening to regulatory submission
Flexible engagement models: Services available as stand-alone projects or as part of a collaborative, integrated drug discovery (IDD) program
Fast turnaround time: Compound dispatch to data upload in 10 calendar days with >95% TAT adherence
World-class infrastructure: The association for assessment and accreditation of laboratory animal care-accredited, large in vivo facility with the latest and most advanced techniques, including Ultra performance liquid chromatography-tandem mass spectrometer (QQQ/Qtrap/Q-TOF) and robotic automation for performing in vitro and bioanalytical assays
Data integrity practices: Fully implemented electronic lab notebooks, laboratory information management systems (LIMS), and good research practices (GRP)
Why Syngene Is the Right Partner for DMPK in Drug Discovery
The right partner for DMPK ADME needs can help reduce compound failure, identify compounds with optimal safety profiles, and minimize drug-drug interactions in later stages. Syngene’s world-class team of scientists drives cost-effective, reproducible, high-quality DMPK data for our clients. With our fast turnaround times, flexibility, and commitment to your project, we can help you achieve a high success rate with your clinical candidates across modalities.
About the Author








