iPSCs in Drug Discovery: Why Quality and Control Matter
The discovery of induced pluripotent stem cells (iPSCs) has opened a new channel for novel drug discovery, disease modeling, and toxicity studies. As much as iPSCs are an attractive choice in the drug discovery space, it is very important to follow best practices for iPSC research in order to maximize their potential.
In this point of view, we discuss the rigorous measures established at Syngene for tissue procurement, iPSC reprogramming, systematic cell banking, inventory management, and quality control for every iPSC batch. These measures are crucial for ensuring the delivery of high-quality iPSCs across application areas to maximize drug discovery success.
Key Considerations for Quality Control
iPSC Cell Morphology as a Quality Indicator
iPSC cells have a distinguished morphology, as shown in Panel A, characterized by compact colonies with a clear boundary and uniform morphology. A routine check of iPSC culture by morphology is the easiest and fastest way to ensure that your iPSC cell cultures are pluripotent. At this stage, it is very important to watch out for spontaneous differentiation of iPSC colonies, as seen in Panel B, where a patch of fibroblast-like cells (shown with red stars) would start to differentiate from one side of the colony, resulting in the iPSC colony no longer having a uniform boundary.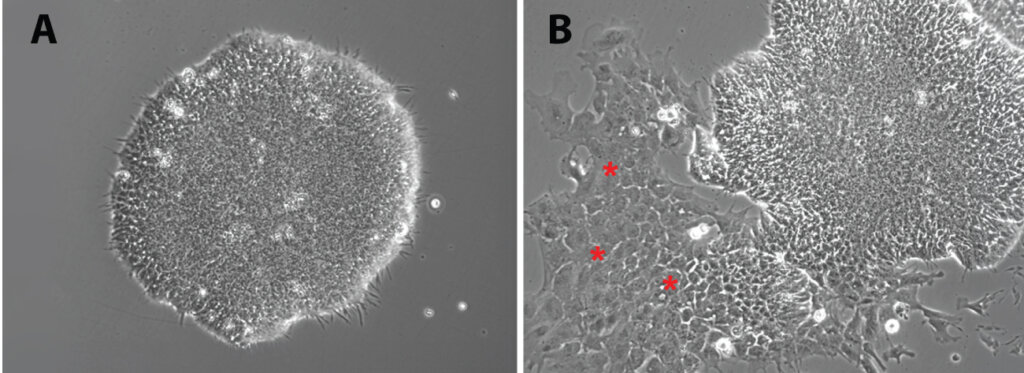
Pluripotency Markers and Immunocytochemistry in iPSCs
It is imperative to check iPSC cultures regularly for expression of pluripotency markers by Immunocytochemistry (ICC) or flow cytometry. Some common pluripotency markers tested include Oct4 and Sox2 (nuclear markers), SSEA4, and TRA-1 60 (cell surface markers). Below is a representative example of a pluripotency check of iPSCs by flow cytometry (A) and ICC (B). Both the assays presented below show that these iPSC cultures are highly pluripotent.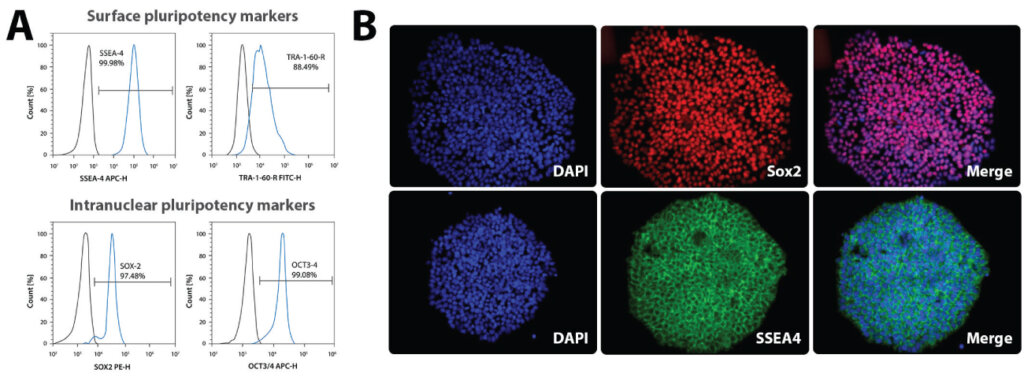
Trilineage Differentiation Potential of iPSCs
As much as it is essential to check the iPSCs/iPS stem cells regularly based on the expression of pluripotent markers, it is crucial to access the differentiation potential of iPSCs into all three lineages (ectoderm, mesoderm, and endoderm). If iPSCs are not fully reprogrammed from the somatic tissue, they may still show high pluripotency but will not differentiate well into all three lineages.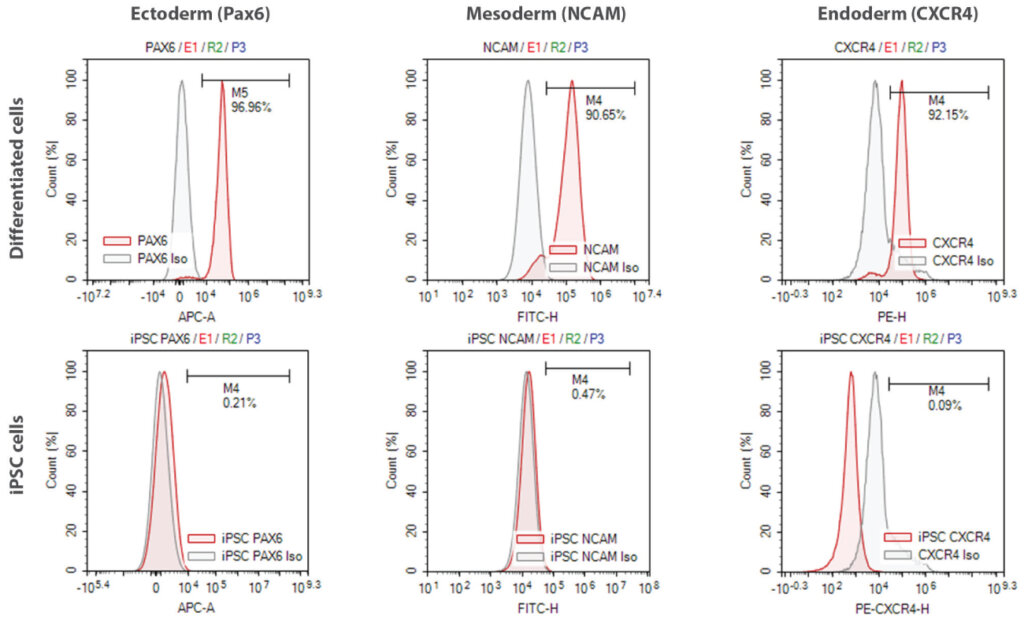
iPSC pluripotent stem cells were subjected to trilineage differentiation analysis, and the differentiated cell lineages were assessed by staining with lineage-specific markers either by flow cytometry (top panel) or ICC (bottom panel). Both the assays show that Syngene iPSCs can successfully differentiate into all three lineages with high purity. As part of quality control, iPSC cells are stained with lineage-specific markers. The absence of lineage-specific marker expression in iPSCs indicates the specificity of the studies.
Genetic Stability and Authenticity of iPSCs
It is vital to check iPSC cultures for any genetic abnormality after reprogramming and at regular intervals while maintaining the iPSC cultures. Karyotyping is one of the standard methods to test for any chromosomal abnormality. Other genetic testing methods commonly used are HLA typing and STR typing to keep the cell line authenticity in check. If these abnormalities are not checked regularly, it could impact the growth kinetics of iPSC culture. Any results produced from poorly characterized iPSCs can be misleading.Sterility check of iPSCs
iPSC cultures must be routinely checked for bacterial, fungal, and mycoplasma contamination. Unlike bacterial and fungal contamination, which are easy to visualize, mycoplasma contamination is a silent killer. The mycoplasma contamination can go unnoticed for days together and could severely compromise cell health and growth kinetics. Syngene has sensitive PCR detection tools and a dedicated mycoplasma testing team to check all the iPSC cultures for mycoplasma contamination regularly. It is crucial to work with myco-free cultures to ensure the quality of the assays performed with the cells.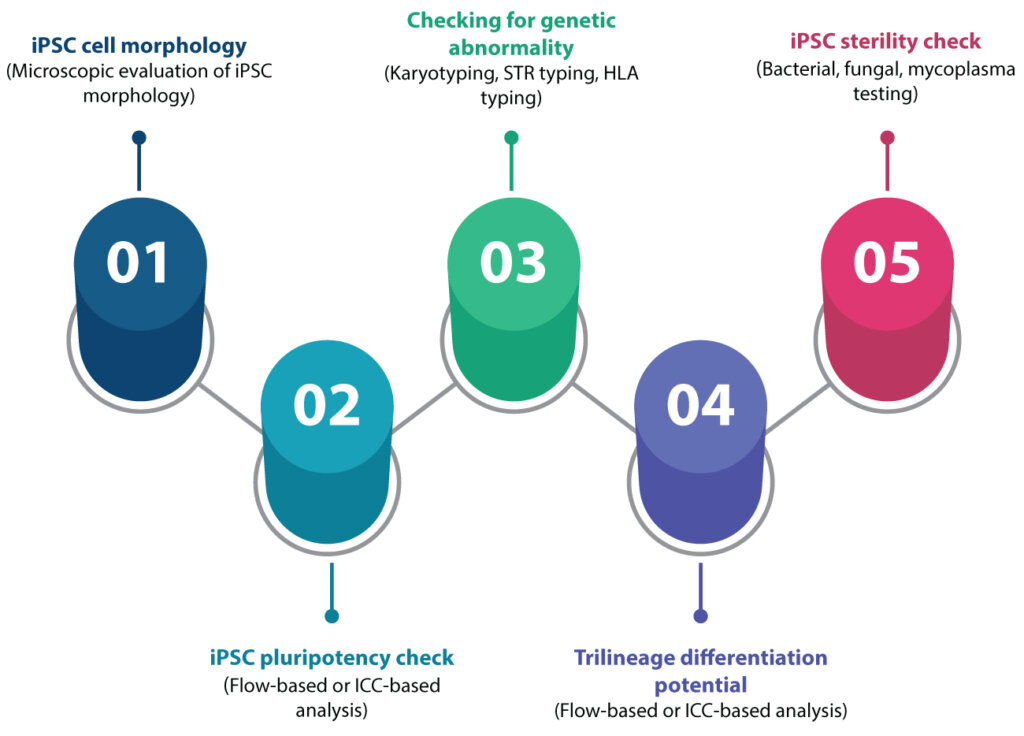
End-to-End iPSC Quality Management at Syngene
Syngene has an effective management system to generate and distribute premium quality iPSCs. The system comprises four sections:
1. iPSC Generation and Tissue Procurement
The first prerequisite for iPSC stem cell generation is to obtain human somatic tissues like PBMCs and skin fibroblasts. Syngene has MTAs with hospitals for the availability of starting material required for iPSC reprogramming. The donor recruitment methods, consent form, and sample collection procedures comply with ethical, legal, and regulatory authorities. We have a methodical screening process to screen donors to ensure the quality of each sample. Syngene has well-established standard operating procedures and technical expertise that guarantee high reprogramming efficiency and generates feeder-free, integration-free iPSC cells customized to customer needs.2. iPSC Characterization and Pluripotency Validation
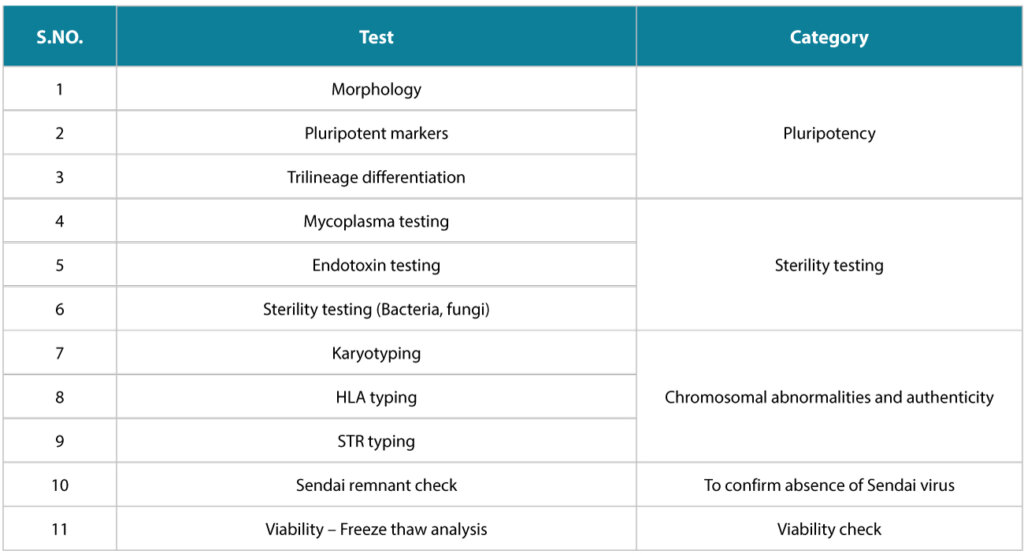
Syngene has an extensive 11-parameter Quality checklist that involves- Pluripotency check,
- Sterility testing,
- Chromosomal abnormality and authenticity
3. iPSC Banking and Traceability
Each iPSC cell line at Syngene has a unique identifier to distinguish it. Each iPSC line nomenclature is linked to the donor identity for easy traceability. For each line, we have a fully characterized master bank and a working bank using all QC parameters. Our automated inventory helps us bank and dispense the vials seamlessly. All our iPSC stocks are stored in vapor phase LN2 storage tanks, the optimum storage condition for iPSC cells. All our LN2 tanks are carefully monitored 24/7 and have auto-refilling in case of temperature fluctuation to ensure high cell viability upon revival.4. Controlled iPSC Distribution
Syngene has a structured procedure to QC the iPSC cell stock before distribution. Clinical outcome assessment (CoA) is provided with every iPSC shipment that contains details of all the QC checks done on every batch of cells. These measures ensure that we deliver high-quality iPSCs without fail.Delivering High-Quality iPSCs to Accelerate Drug Discovery
Using iPSC stem cells for biomedical research is challenging yet rewarding. iPSC-based research has opened several avenues for treating multiple diseases in the drug discovery space. Although there are many approaches to reprograming and maintaining iPSCs in culture, what remains crucial is maintaining the quality of the iPSCs. Syngene has an experienced and dedicated iPSC team, including a state-of-the-art iPSC facility. Over the years, we have delivered several successful preclinical projects using iPSCs. Our systematic end-to-end procedure comprises required MTA, permissible donor consent, unique identification of each line with traceability to the respective donor, quality control of each iPSC line, batch CoAs, regulated inventory, and data management. This ensures the best quality iPSCs regardless of application area, making us a reliable partner for iPSC-based services to accelerate drug discovery.
About the authors








