Introduction
According to research, 85% of new drugs fail during clinical trials (Phase-1 to FDA approval). The reasons for failure include poor solubility, life-threatening or other undesirable side effects, poor biodistribution by the proposed clinical route of administration, poor efficacy in early clinical trials, and so on.
For a molecule to progress smoothly from early stage discovery to the investigational new drug (IND) phase and further to successful clinical trials, the data from preclinical trials must be accurate, reliable, and based on the best suitable and comparable model available to the target population. This means the IND or drug product must undergo a series of robust tests and experiments as per the focused indication and regulatory guidelines (FDA/EMA etc.).
In this point of view, we discuss how Syngene’s expertise in In vivo Safety /Tox studies can help move your molecule from early discovery to IND stage successfully. With 27 years of scientific experience, working across 400+ global clients, we have been able to create a roadmap for IND-enabling preclinical studies, supported at each stage by a strong supply chain, logistics, program management, and data security. Read on.
Syngene’s capability in preclinical Safety/Tox studies
Syngene’s integrated drug development and assessment capabilities put it at the forefront of discovering new therapies for various diseases. We can support you from the very start of your drug development journey to the clinic and further to commercialization. We have extensive experience working in this space, including in the field of IND-enabling safety and toxicity studies. So far, we have supported 400+ global clients from early discovery to development across multiple targets.
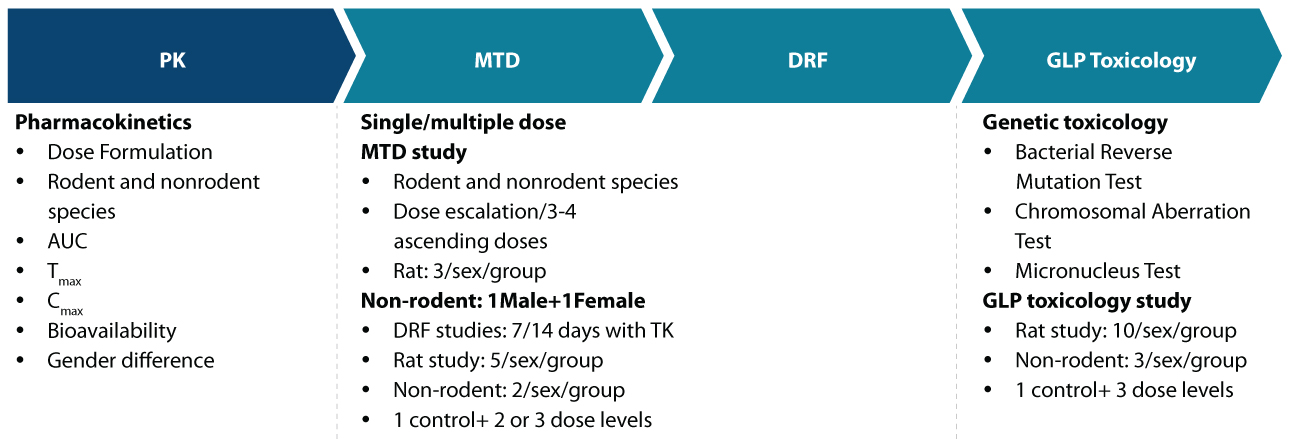
Our expertise in Safety/Tox studies allows us to guide you through the various requirements leading up to IND submission (Figure 1). These include:
Assessment of pharmacokinetics such as dose formulation in rodent and non-rodent species, the bioavailability of the molecule, and assessment covering gender differences
Maximum tolerated dose (MTD) studies and dose range-finding (DRF) studies across rodent and non-rodent species
Assessment of genetic toxicity using bacterial reverse mutation test, chromosomal aberration test, and micronucleus test. Also, GLP toxicology studies in rats and non-rodents.
We also have a range of species for testing target molecules. While a majority of the studies recommend using rats and dogs, other species can also be used if data suggests that they are more biologically relevant. For clients that prefer the use of non-rodent species, we offer preclinical studies which can be conducted in non-human primates such as minipigs.
Pivotal GLP tox study
A typical study design for pivotal GLP studies for rodents and non-rodents involves a dosing phase of 28 days, followed by a recovery phase of 14 days or 28 days. The rodents/non-rodents are divided into four groups (control, low dose, mid-dose, and high dose), each consisting of an equal number of males and females with standard observations/endpoints. Toxicokinetic is integrated into the pivotal GLP tox study. The parameters observed include morbidity and mortality (daily checks); pre and post-dose clinical signs (pre and post-dose); ophthalmic examination; bodyweight; food consumption; clinical pathology (main groups and recovery groups); necropsy and gross examination; histology of all organs including injection site(s), if any; dose formulation concentration analysis and bioanalysis and toxicokinetics.
Safety Pharmacological studies
We conduct Pharmacological studies using the Irwin test, Respiratory Function test, Cardiovascular Safety Pharmacology test, and in vitro hERG assays.
Irwin test: We conduct this study to test the pharmacological effects of target molecules on the central nervous system in rodents. We use it to assess the functionality and toxicity of chlorpromazine, triadimefon, trimethyltin, and acrylamide. The test is also used to assess many bodily functions, such as respiratory pattern, mobility, gait, salivation, pupil reflex, and motor activity (Figure 2)
Respiratory function test: A special plethysmograph chamber is used to assess the respiratory properties of rodents. The parameters monitored include Ti-inspiration time, Te-expiratory time, PiF-peak inspiratory flow, PeF-Peak expiratory flow, TV-Tidal volume, MV-Minute volume, F-Respiratory rate, RT-Relaxation time
Cardiovascular safety pharmacology: Telemetry [Jacketed/Invasive] using non-rodents
In vitro human Ether-a-go-go Related Gene (hERG) assay: Patch clamp
Biologics Studies
To test a target molecule in an animal model, we pay special attention to the following:
Selection of relevant animal species
Studies are done in relevant species, i.e., species that demonstrate pharmacological effects
With biologics, non-human primates (NHPs) are often the only relevant species available for testing
If a second species is relevant, we evaluate them as well
No relevant animal model other than transgenic strain expressing human receptor is considered for studies
Dose, route of administration, and treatment regimen
High dose, 10X over maximum exposure, tested to establish an adequate safety margin.
Route/frequency of administration kept the same as that proposed for clinical use
Conducting pivotal 28-day repeated dose toxicology study in relevant animal species
Vaccine Studies
Our vaccine study design comprises a 43-day dosing phase, followed by a 28-day recovery period. Animals are assessed for clinical pathology, organ weights, gross pathology, histology of organs, immunogenicity, local tolerance, and other clinical signs.
We also conduct vaccine prenatal developmental toxicity study design covering the following:
Clinical signs, local tolerance, body weight, and food consumption
Cesarean section: Gestation day 20
Maternal data: Uterine weight, # corpora lutea, implantation sites, early and late resorptions, pre-and post-implantation losses
Litter data: Total no. of fetuses (live and dead), litter weight, Sex ratio (M: F)
Fetal evaluations: External, visceral, and skeletal malformations. Classified as normal variants, minor anomalies, and major malformations
Immunogenicity: Prior to dosing and at termination
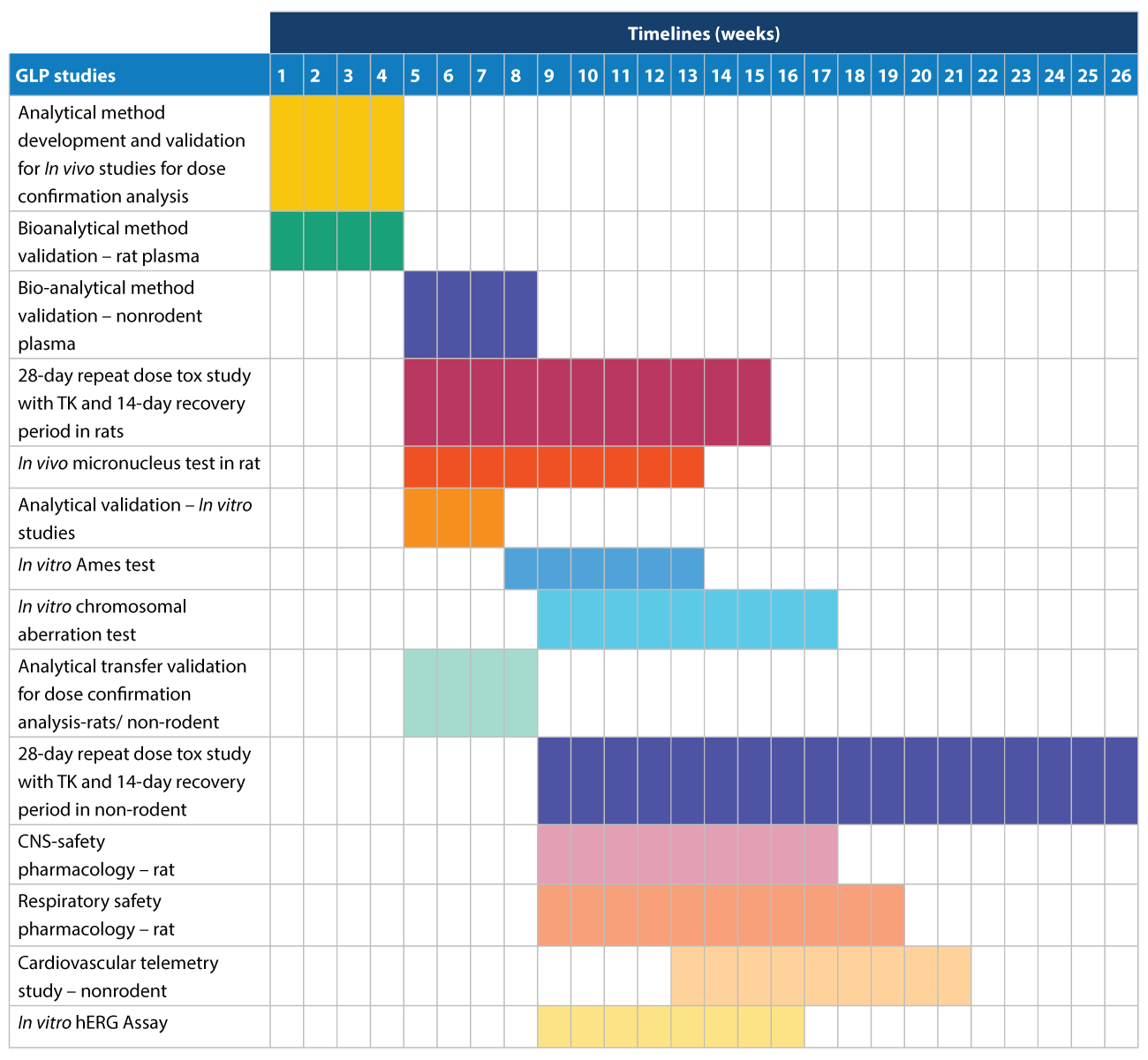
IND GLP Tox and Safety Pharmacology studies
We offer the following IND GLP Tox and Safety Pharmacology studies across a 26-week timeline (Figure 2):
Conclusion
Syngene has significant expertise in preclinical studies across species (rodent/non-rodent) and a deep understanding of the molecular assessment process. Our studies are performed in compliance with good laboratory practice and good scientific practices (GLP/GSP) to ensure reliability and reproducibility of results that satisfy stringent regulatory guidelines (i.e., FDA/EMA). By partnering with us, biopharma companies can greatly increase their chances of moving their molecules from Target to IND successfully. Further, Syngene has also significantly optimized the various steps involved in taking a molecule from lab to human trials and commercialization. With this, biopharma can reap the benefits of accelerated timelines for synthesizing new drugs.
About the Author








