Introduction
Whether it is measuring the concentration of a biologic and its target in a human matrix or characterizing anti-drug antibodies from a clinical trial, the responsibility of generating meaningful, clinically relevant assay data lies with the bioanalytical scientist.
Analyzing samples collected from subjects participating in clinical trials is crucial to the clinical trial process. Bioanalysis of these samples provides essential data on various endpoints to assess the pharmacokinetic (PK) profile of large molecule investigational medicinal products and monitor their safety and efficacy. Further, sample analysis or evaluation must be performed per acceptable standards. This ensures the data reported is reliable, accurate, and safe for patients.
To ensure quality data, any large molecule bioanalytical team needs to develop and validate fit-for-purpose immunoassays compliant with good laboratory practices (GLP) or good clinical laboratory practices (GCLP) – so that the data generated is meaningful to clinicians for further investigations and correlation.
In this viewpoint article, we discuss how to establish the connection between clinical and bioanalytical teams to ensure successful outcomes of GLP/GCLP large molecule bioanalytical (BA) sample analysis.
Clinical Trials team Vs. Bioanalytical team
In a complex communication network, the GLP/GCLP immunoassay team should work with GCP teams of PK scientists, site specialists, protocol
groups, central laboratories, and clinicians to better understand the clinical trial features and requirements for assay design. A breakdown in
this complex communication network would lead to assays not being developed for their intended purpose and result in invalid, irrelevant data
being generated. This could result in the objective of the clinical trial itself being nullified because of insufficient data.
Given below are some examples of the critical information needed from the Clinical team, which could have assay or method-related
implications if not received on time.
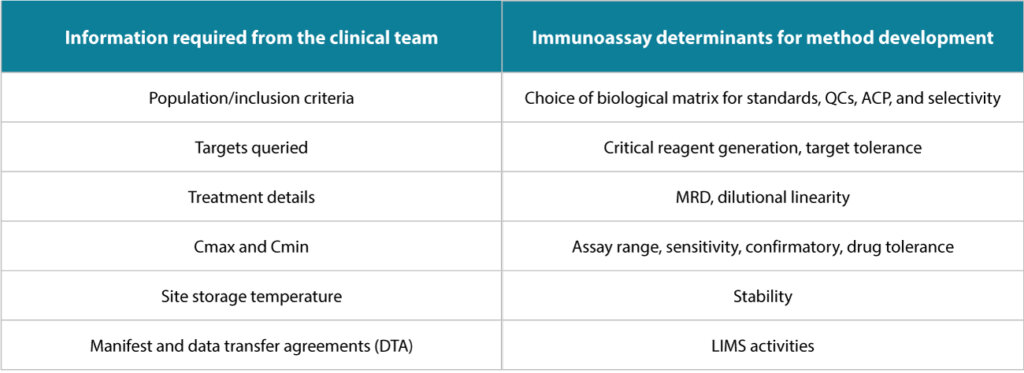
The Clinical team is responsible for designing, conducting, performing, monitoring, auditing, recording, analyzing, and reporting clinical
findings in a compliant manner. This ensures the data and results are credible and accurate and the rights, integrity, and confidentiality of the
trial subjects are protected per GCP guidelines.
On the other hand, the bioanalytical team is responsible for measuring the concentration of the biologic and its target in the human matrix, the
characterization of anti-drug antibodies from a human trial, and generating meaningful, clinically relevant assay data per GLP/GCLP principles
post-dosing.
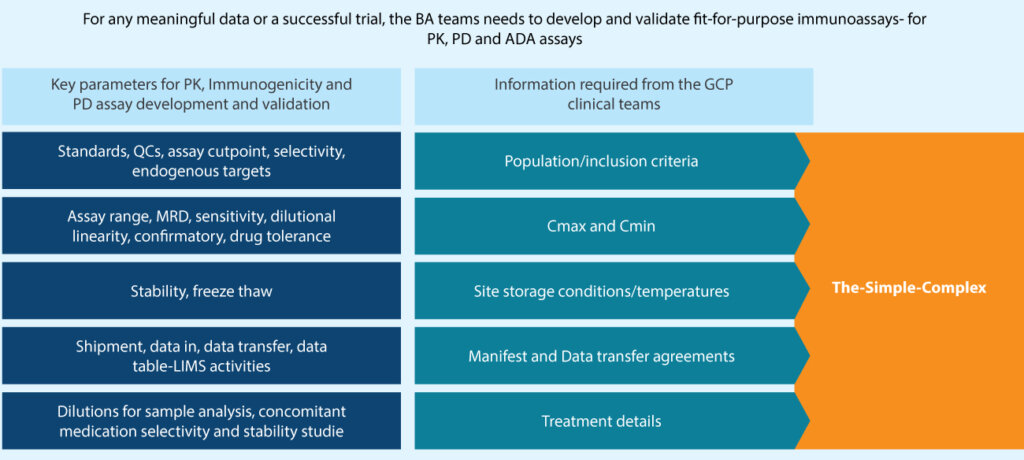
While both teams appear to have completely different objectives, for any meaningful data or successful trial, both these teams need to work
together in a coordinated manner to achieve the final goal. For bioanalytical methods to be validated as fit-for-purpose, scientists must work
closely with the clinical team to obtain critical clinical trial design data. The requirements for validation have been illustrated below (Figure 1).
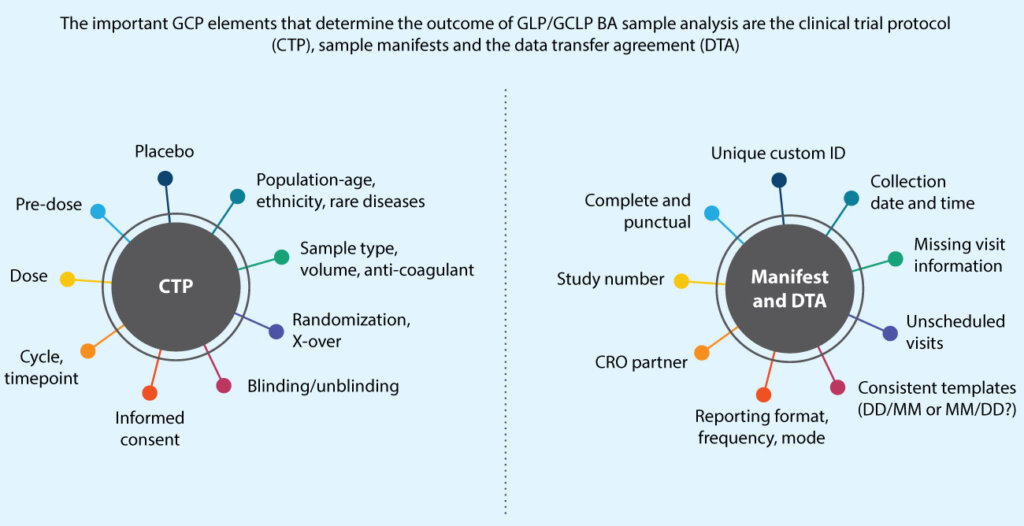
Key GCP elements during the reporting phase that determine data presentation for bioanalytical sample analysis are presented below (Figure 2).
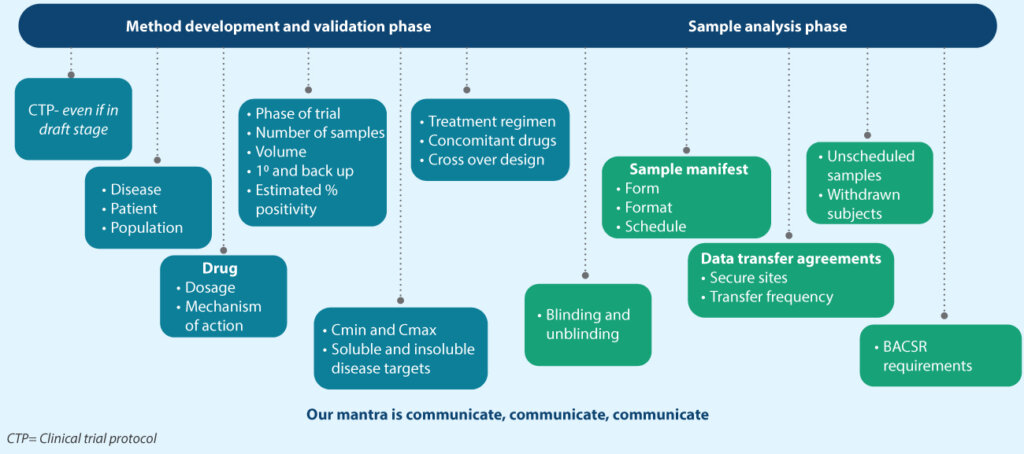
Syngene’s processes to ensure all GxP needs are met
Over the last decade and a half, Syngene has incorporated new processes and workflows to meet all clinical trial objectives with regard to bioanalysis (Figure 3). We have recognized that a strong communication flow from the beginning of a project is vital for any program to be successful. Standard operating procedures (SOPs), guidance, training, and multiple pre-study meetings with numerous stakeholders have helped us seamlessly develop and validate fit-for-purpose methods and clinical bioanalysis. Besides revising SOPs and processes, we have also built and validated special software to reformat sample manifests to make them eligible for laboratory information management systems (LIMS) import.
Syngene’s processes to ensure all GxP needs are met
A biopharma company partnered with Syngene for large molecule bioanalysis studies. As part of the study, we received multiple shipments of samples from different parts of the world within 5-7 days from the client’s clinical trial CRO partner. After successful method validation, we were ready for sample analysis. However, the sample manifests sent by the clinical team came in different formats, which our bioanalytical LIMS system could not read (Figure 4).
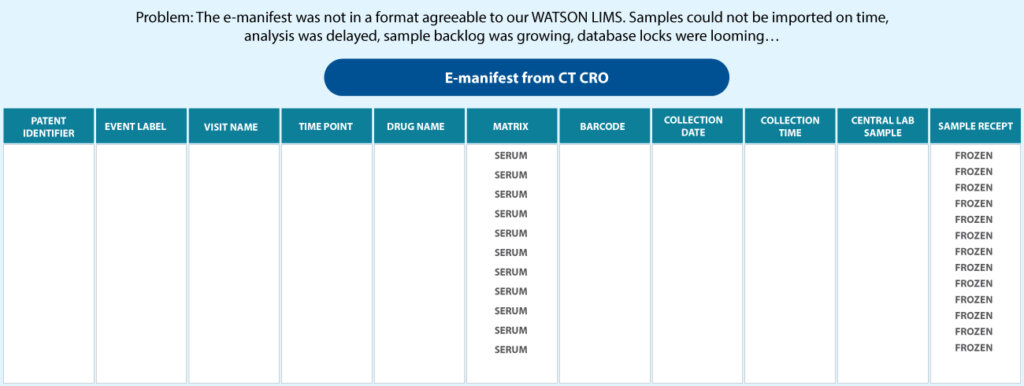
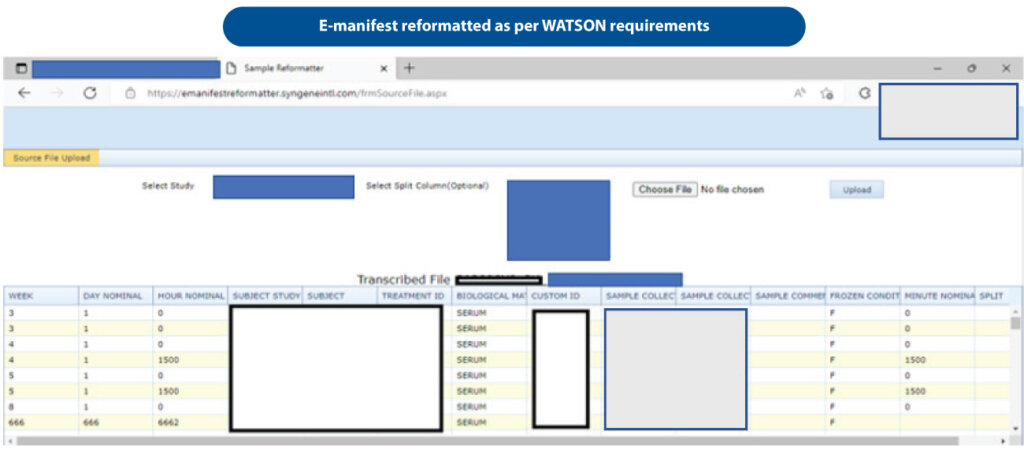
Discussions between the two teams were not fruitful. Despite several attempts, the CRO Clinical team could not address our manifest needs, resulting in a considerable initial backlog of samples for analysis. Since samples could not be imported on time, the study was delayed. Sample backlog began to grow, leading to the possibility of missing database lock timelines. To overcome this challenge, Syngene developed an e-manifest reformatter that could read and reformat various sample manifests into a LIMS readable format (Figure 5).
Syngene’s processes to ensure all GxP needs are met
Following this, the study manifests were reformatted into a LIMS-readable format so that sample analysis could proceed per study requirements. The software solution not only saved the clinical trial from failing but also played a crucial role in securing regulatory approval for the molecule. Using this learning, our process now includes ‘manifest alignment’ with clinical trial CROs well in advance.
Conclusion
Clinical research aims to ensure safe and efficacious treatment for patients. However, the GLP/GCLP bioanalytical and GCP clinical teams tend to work with entirely different objectives.
The complex connection between the two teams needs to be established, managed, and nurtured as well. At Syngene, we have a simple mantra for ensuring this connection – communicate, communicate, communicate. Timely and regular communication helps us achieve clinical trial objectives smoothly. In cases where matters cannot be sorted despite regular communication with the clinical teams, it is advisable to deploy an internal strategy to overcome the problem.
Syngene’s GCLP/GLP large molecule bioanalytical laboratory develops, transfers, and validates PK, ADA, Nab, PD, and biomarker methods in support of safety efficacy endpoints of biologics. The laboratory works with biomanufacturers from all over the world and receives samples from across geographies.
In the last 17 years, The laboratory has validated more than 300 bioanalytical methods in compliance with current international regulatory requirements and guidance.
About the author








