Introduction
Chimeric Antigen Receptor T-cell (CAR-T) therapy is a cell and gene therapy modality where a patient’s immune T cells are genetically modified in the laboratory to bind to cancer cells. This is done by engineering a cell-membrane antibody fragment in such a way that it recognizes a specific protein expressed only in cancer cells. Once injected into the patient, the CAR-T cells stimulate the immune system through internal signaling domains connected to the antibody fragment, allowing T cells to identify and kill the cancer cells specifically. The T cells are typically derived from the patient (autologous) or other donors (allogeneic).
Research into this unique modality has been gathering momentum in recent times. Since 2017, six CAR-T therapies for blood cancers have been approved. Despite the high costs ($0.5M/patient), manufacturing bottlenecks, and safety concerns, the cell and gene therapy market is projected to grow at a CAGR of 29.8% from $8.44B in 2023 to 88.2B in 2032 (Precedence Research, 2023).
In this viewpoint article, we explore the top challenges in developing CAR-T therapy for treating cancer and how leading CRO-CDMO Syngene supports clients in bringing this therapy to patients faster.
Introduction
Tumor Antigen Specificity: The success of CAR-T depends on identifying a specific antigen (e.g., CD19 in B-cell malignancies), limiting its applicability to certain cancers.
Toxicity and side effects: Cytokine release syndrome (CRS) is one of the most common side effects of CAR-T therapy. CRS can cause fever, low blood pressure, and multi-organ dysfunction, requiring careful monitoring and sometimes intensive care.
Personalized nature of treatment: CAR-T therapy is personalized, requiring the collection of a patient’s own T-cells, which are then genetically engineered and expanded before reinfusion. This is time-consuming and costly, besides leading to T cell exhaustion.
Targeting solid tumors: CAR-T therapy has shown remarkable success in hematologic malignancies like leukemia and lymphoma but has been less effective in solid tumors. One of the main reasons is that solid tumors create an immunosuppressive environment, making it difficult for CAR-T cells to function effectively.
Manufacturing process: Producing plasmid DNA (pDNA) is a crucial step in developing CAR-T therapies, as it is used to insert the genetic code that enables T-cells to express the CAR. However, producing pDNA itself presents several challenges, such as meeting high purity requirements, yield, scalability, regulatory compliance, etc. Variations in the manufacturing process can also affect the efficacy and safety of the therapy
CAR-T discovery and development at Syngene
Syngene has strong capabilities in CAR-T discovery (Figure 1). Our antibody discovery team can identify novel binders to specific cancer cell antigens using a variety of platforms, including library screening, hybridomas, or single B cell culture. Our genomics team then engineers the CAR construct by linking the Ab fragment to internal signaling domains that activate the T cells (plug-and-play vectors). Our assay biology team uses lentiviral or RNA delivery systems to introduce the CAR into primary human T cells, co-cultures these T cells with cancer cells, and evaluates the efficacy, antigen-specificity, and cytokine release through sophisticated assay models (Figure 2). These experiments helped our partners rapidly identify the best CAR with the highest cancer-killing ability and with minimum or no cytokine storm when transduced into T cells. We have extended this work to T cell subtypes and other immune cells. Our in-house PBMC banking allows us to test the efficacy and safety of CAR-T constructs in a wide variety of donors. We also have the capability to evaluate CAR-Ts in humanized mice models.
For development, Syngene offers GMP plasmid manufacturing of the various lentiviral vectors (see below). We also have access to a 10L batch for lentivirus scaleup through our partnership with Immuneel Therapeutics Pvt Ltd.
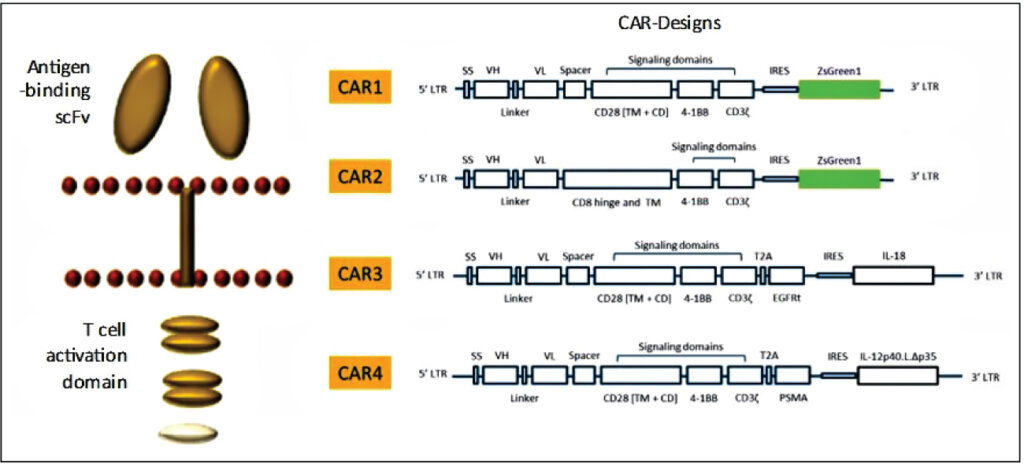
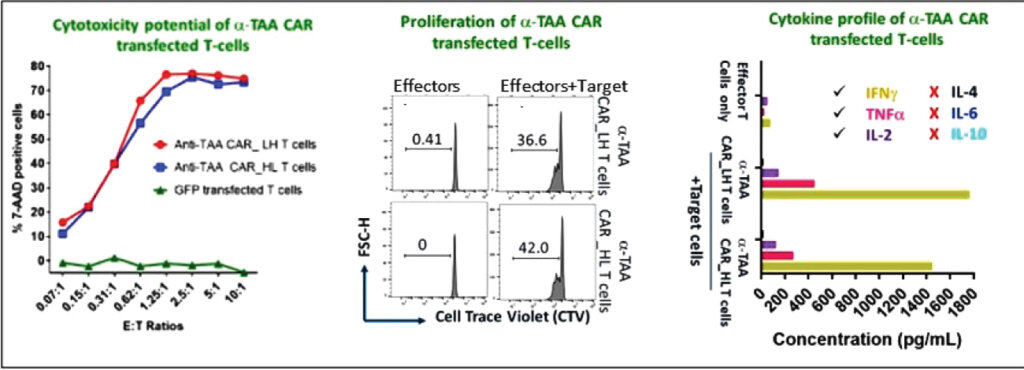
Allogeneic CAR-Ts
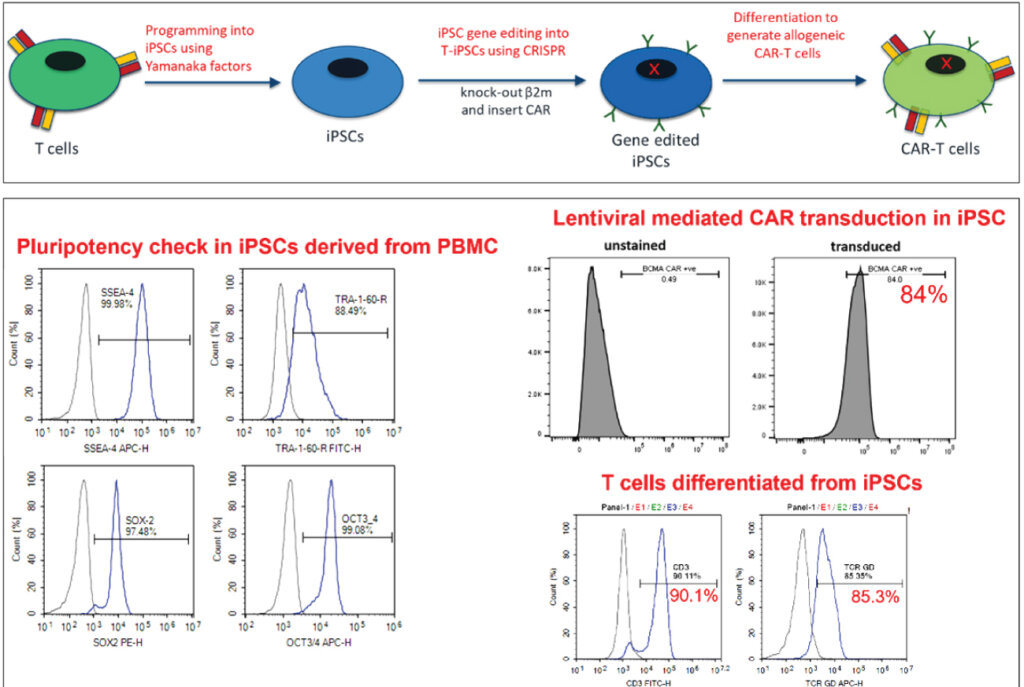
Engineering each patient’s T cells with CAR constructs in the lab and putting them back into patients is a lengthy process leading to T cell exhaustion. The future of CAR-T therapy is anticipated to be based on off-the-shelf, donor-derived CAR-Ts that can be directly given to patients. These can be engineered, frozen, and administered to multiple patients with the same cancer type. The process involves taking T cells from multiple donors, programming them into iPSCs using Yamanaka factors, followed by gene editing to reduce immunogenicity, introducing the CAR construct, and finally re-differentiation into T cells that now have the CAR. Using the above procedure, we have successfully generated allogeneic CAR-T cells using a subset of T cells (Figure 3). The methods developed at Syngene have been successfully used by partners to scale up the cells for direct use in patients.
T cell receptor T (TCR-T) therapy
Although CAR-T therapy has shown remarkable results for liquid tumors, its efficacy in solid tumors is still in its infancy. TCR-T is a therapy where the endogenous TCR in T cells is replaced with an engineered TCR-T and represents a promising clinical alternative to treating solid tumors. We have high capability in TCR-T therapy. Using AAV methodology, we can knock out endogenous TCR and introduce antigen-specific TCR in T cells. We can also do combination therapies, e.g., CAR-T/TCR-T, along with knock-out of immune checkpoint inhibitors and determine the efficacy of combination immunotherapies using multiple read-out assays, including cytotoxicity, T cell proliferation, and cytokine profiling.
Gene therapy
The first FDA-approved gene therapy for sickle cell anemia discovered by Vertex Pharmaceuticals through gene editing technologies has opened the field of gene therapy to treat rare disorders by correcting defective genes using CRISPR gene editing or through specific antisense oligonucleotides. We have acquired the CRISPR license through ERS Genomics and have performed numerous gene editing studies for our clients by knocking out or knocking in genes for target validation, mechanism of action, and proof-of-principle studies. CRISPR-based gene knock-out in iPSCs was instrumental in developing the allogeneic CAR-T platform at Syngene. Combined with our AAV and lentiviral capabilities in discovery, this enables us to nimbly evaluate cellular and physiological consequences of gene therapies in multiple primary cell types and animal models. In addition, our capabilities in antisense oligonucleotide (ASO) synthesis and antisense cell biology position us to screen for the best ASOs for potency, in vitro and in vivo efficacy, and in vivo toxicity.
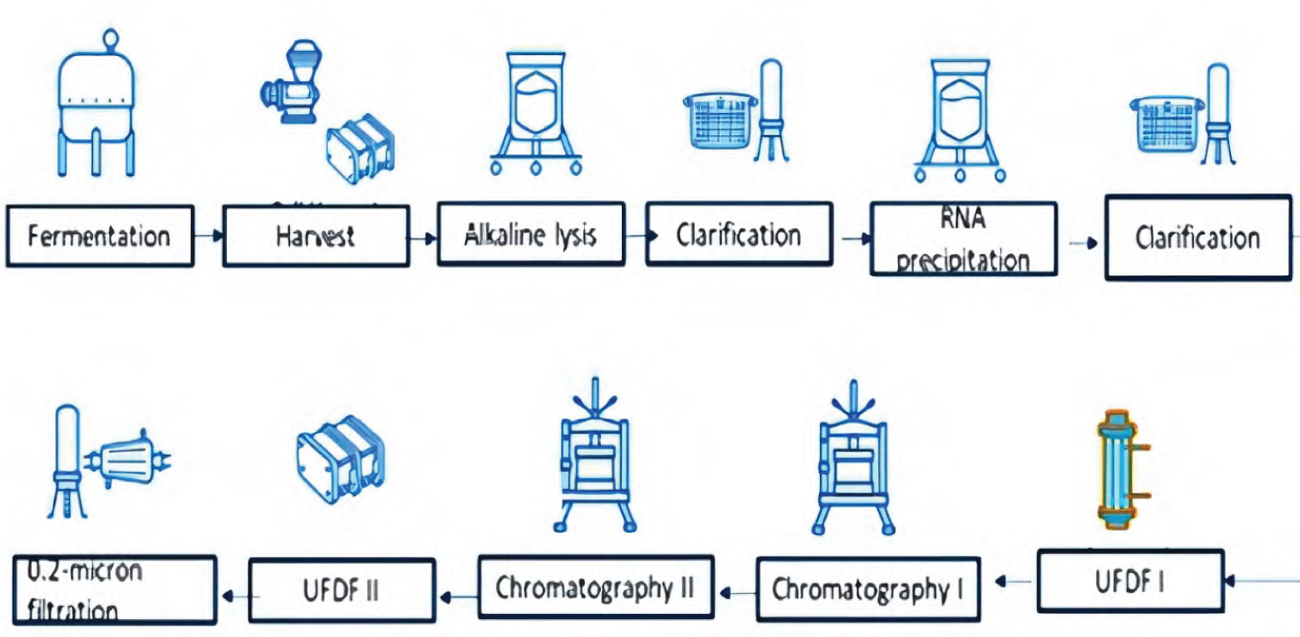
pDNA process flow
At Syngene, we have established a robust platform process for both upstream and downstream operations. Our upstream process delivers high titers (>500 mg/l) for medium-high copy number plasmids and has been successfully scaled up to 50L in a stainless steel bioreactor for global clients. Additionally, our downstream processes are equipped with state-of-the-art equipment capable of high recovery (up to 40%) and producing high-quality pDNA (>90% supercoiled). Our process is meticulously designed to meet regulatory guidelines (Figure 4).
Summary
With nearly 9.7 million cancer-related deaths in 2022, cancer is among the leading causes of death worldwide. By 2040, the number of cancerrelated deaths is expected to rise to 15.3 million (US National Cancer Institute). Given the emotional and financial toll on families affected by this disease, the race to find a cure can never be fast enough. As research in CAR-T cell therapy evolves, the endeavor will always be to find new ways to overcome current limitations, expand applications, and extend benefits to other cancer types, while optimizing safety, affordability, and long-term success. Already, progress has been made in improving CAR-T persistence in solid tumors, targeting other immune cells (CAR-NK, CAR-macrophages), and controlling cytokine release syndrome (CRS) and toxicity to limit adverse effects. By partnering with a CRDMO like Syngene, which has end-to-end capabilities, advanced technology, and an innovation mindset, biotechs can bring much-needed cancer therapies to patients faster
Customer Speak








