Introduction
Animal testing has been used extensively for the prediction of human toxicity. In addition to the cost and time involved, ethical considerations and increasing restrictions on animal testing (e.g., Europe’s 2013 prohibition on animal testing for cosmetic ingredients) have made the development of alternative methods to reduce and replace the number of animals used in toxicological tests, imperative.
Moreover, the assumption that animal models can accurately predict toxicity has been acknowledged as unreliable. A review of 221 animal experiments found that they agreed with human outcomes only ~50% of the time. The failure rate of clinical trials due to unanticipated toxicity also raises concerns about the reliability of in vivo testing [1]. In this article, we discuss how Syngene’s Computational & Data Sciences unit offers toxicity assessment across various levels of complexity to enable a holistic, mechanism-driven approach to toxicity assessment.
In vitro toxicology
Various in vitro assays are being considered as alternatives to animal testing for toxicity assessment. While they are key to decreasing attrition of candidate molecules through the discovery and development process, in vitro methods have multiple challenges in systemic toxicity assessment. Cell systems can act as simplified representatives of in vivo environments but fail to accurately account for system-level effects (i.e., effects resulting from metabolism in the liver or other tissues).
The in vitro kinetics in the culture medium cannot mimic the actual chemical kinetics that cells in the target tissues experience under real-world exposure scenarios [2]. Multiple assays are needed to capture the effect of a chemical compound and evaluate each toxicity marker, as these measure pre-defined endpoints based on an understanding of the physiological processes [3].
For instance, cell viability (MTT assay), necrosis (ATP depletion), oxidative stress (GSH measurement), steatosis (fatty acid synthase activity), and cholestasis (BSEP transporter) in hepatotoxicity assessment. Thus in vitro toxicity testing methods cannot accurately represent the target organism as they fail to capture organism-level effects. This shortcoming is driving the need for new methods for in vitro-in vivo toxicity correlations (IVIVC) and systems-level safety assessment.
Computational toxicology
Computational methods at various levels of complexity have been developed to predict toxicity outcomes. These include:
- Methods that infer toxicity from chemical structure
- Toxicity signatures derived from omics
- Systems models for mechanistic assessment of toxicity and IVIVC
A lack of appropriate data has previously limited the development and applicability of computational approaches. With the advent of big data repositories and the development of new algorithms, in silico models of toxicity are becoming more broadly applicable, enabling complex mechanistic insights and complementing other approaches to develop a holistic picture of in vivo response.
Computational toxicology
One of the most widely used methods in computational modeling focuses on inferring toxicity at the molecular level using structure-activity relationships.
This approach allows rapid detection of potentially hazardous substances. Structural alerts use databases of simple relationships between molecular features and toxicity derived from known toxicants to flag safety concerns. They are interpretable, and 80% of pharmaceuticals that exhibit residual toxicity contain structural alerts. The presence of a structural alert does not always correlate with a biological response. Steric hindrances, metabolites, or bioavailability may ameliorate the response. Read across methods work on the premise that similar compounds exhibit similar activities.
The test compound is compared with a database of compounds with known toxicity profiles using structure- or property-based similarity. This method is transparent, easy to interpret, and the most common method in REACH dossiers. Quantitative structure-activity relationships (QSAR) apply statistical/machine learning models to predict toxicity endpoints of interest. While QSARs are becoming more effective in toxicity prediction, machine learning models are usually black boxes, not amenable to interpretation. Read-across QSAR methods have been developed to make them more interpretable [4].
All these methods have limitations, including:
- Limited applicability of predictions to the chemical space of the data
- No accountability for concentration and time dependencies
- Lack of mechanistic correlation with the intrinsic biological response
- QSARs can quantify the result of an assay but not complex in vivo outcomes
Elucidating toxicity using omics expression signatures
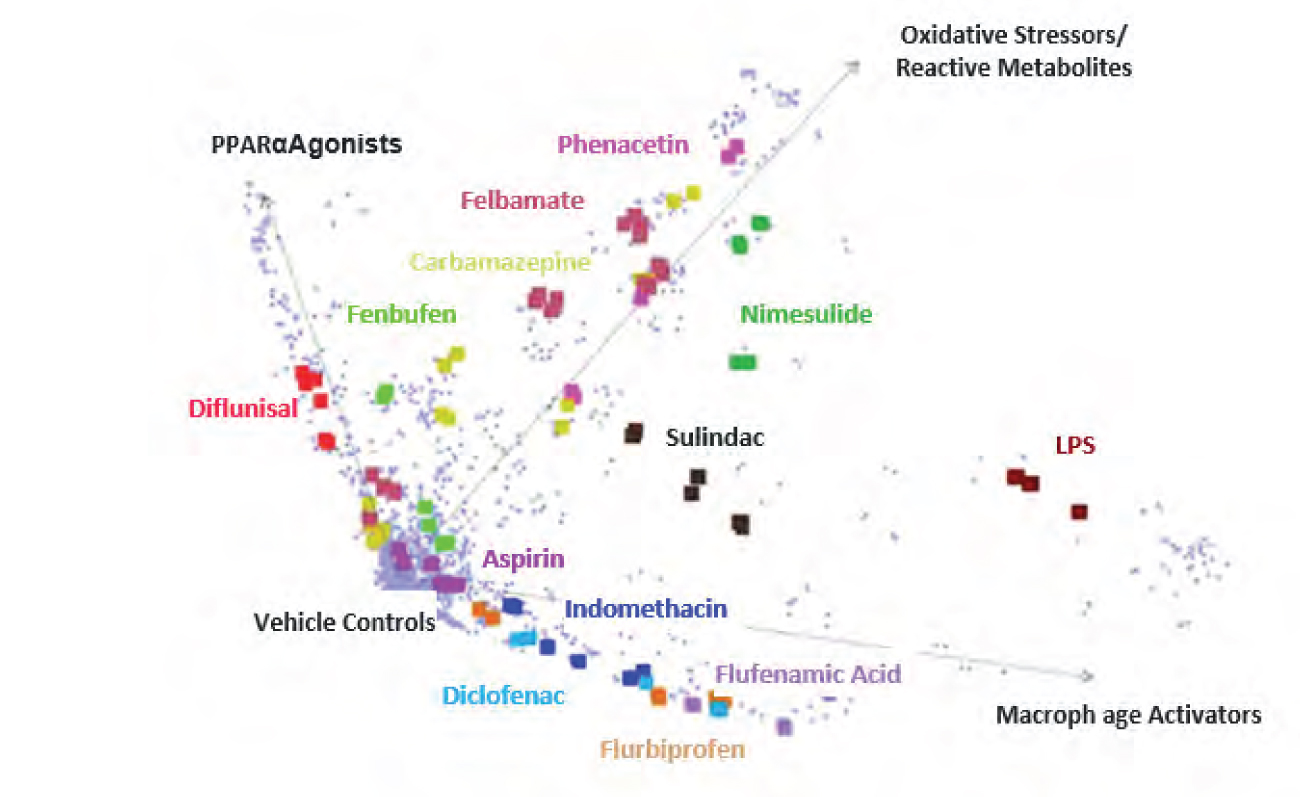
Toxicogenomics can be defined as the application of omics methodologies to elucidate change in cellular response in terms of genes, proteins, and metabolites upon exposure to toxicants. Signatures derived from gene expression can identify toxicity and be linked to biological information for a mechanistic understanding. These approaches can bring out genetic variation in toxicity response and predict idiosyncratic toxicity. An analysis of rat liver gene expression response to compound treatment led to the identification of hepatotoxicants [5]. This approach could also segregate the hepatotoxicants based on their mechanism of action, PPARα agonists, macrophage activators, and oxidative stress/ reactive metabolites using a 70-gene signature.
Systems models enabling quantitative, mechanistic assessment of exposure-dependent toxicity
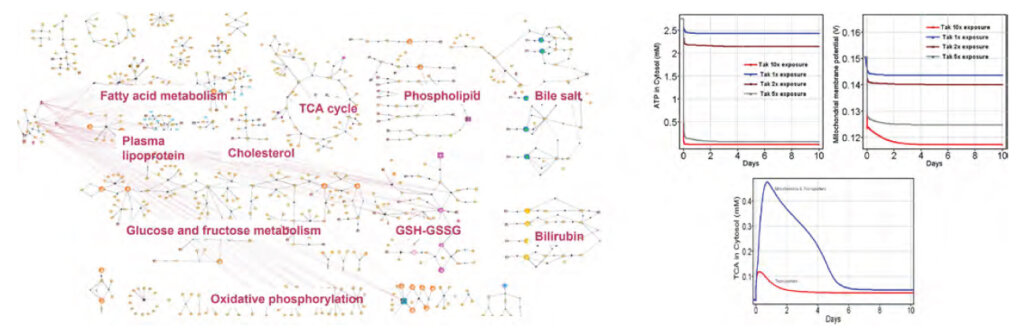
Systems models simulate the outcome of a biological system when it is perturbed by a xenobiotic. They provide mechanistic, time, and concentration-dependent quantitative insights, including in vitro- in vivo correlation (IVIVC). These models are built by mapping key components of a biological system and setting up differential equations for each component to simulate the behavior of the system. A chemical’s effect on the system can then be simulated by using enzymatic changes in assays or changes in expression as input. Syngene’s virtual liver platform HepToxTM is an example of a systems model that can simulate drug-induced liver injury (DILI) in rodents and humans from in vitro measurements.
As discussed above, in vitro and in vivo toxicology assessments conducted in isolation often fail to capture the effect of a compound on a human being. This was seen in the case of fasiglifam (TAK875), a GPR40 agonist that was terminated in Phase III clinical trials due to drug-induced liver injury concerns [6]. Syngene HepToxTM simulations predicted that TAK875 caused mitochondrial dysfunction leading to adenosine triphosphate (ATP) depletion. This further led to a reduction in bile transporter activities resulting in the accumulation of bile salts and inducing cholestasis (Figure 2). This illustrates the effectiveness of systems modeling approaches in bridging the gap between in vitro measurements and human outcomes.
Adverse outcome pathways
An adverse outcome pathway (AOP) is a mechanism to identify and link the sequence of molecular and cellular events required to produce a toxic effect on exposure to a toxicant [7]. An AOP begins with a molecular initiating event resulting from an interaction of a perturbagen and an organism. This results in a cascade of key events which characterize the progression of toxicity. These key events are typically seen at the cellular/organ level. This ultimately results in adverse outcomes in the organism, such as disease, and organ damage.
AOPs provide a holistic outlook on molecular activity, all the way to the manifestation of toxicity by the organism by:
- Integrating information from diverse sources, including in vitro assays, genomics, biological knowledge, chemical information, and in silico predictions
- Providing biological context to experimental data and predictions
- Suggesting mechanism-of-action-based insights to develop and improve testing strategies
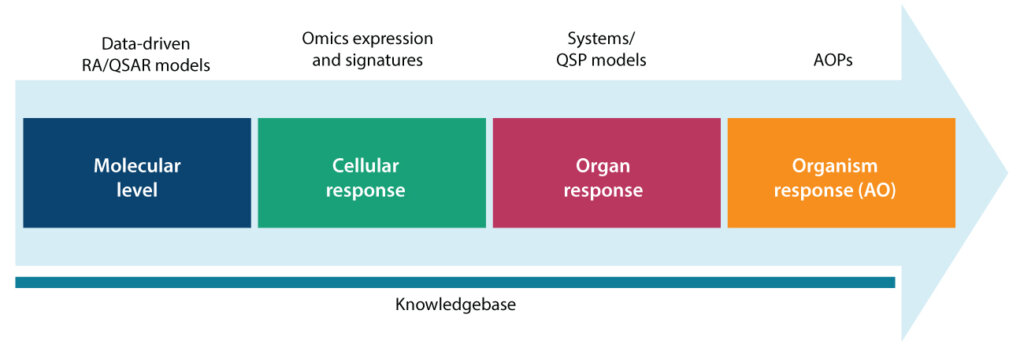
Figure 3 depicts the manner in which we have implemented our data-driven AOP model enabled by computational methods. A knowledge base integrates existing knowledge about toxicants, chemicals, genes, pathways, disease, and biological systems to create a knowledge network that helps rationalize in silico/in vitro data and establish the pathway of adverse outcomes. Methods like QSAR that infer adverse outcomes from a molecular structure can be used for a molecular-level view of toxicity. Expression signatures can provide a cellular-level view of the adverse outcome. Finally, system models can use in silico and in vitro measurements to simulate adverse events in the organ and organism. Syngene has developed AOPs for hepatotoxicity and skin sensitization. Building on these, we plan to develop a comprehensive organ toxicity assessment platform.
Conclusion
Although animal experiments have been the traditional gold standard for toxicity testing, there are shortcomings in their correlation with adverse human outcomes. With ethical, commercial, and legislative considerations, there is a need for a paradigm shift toward the active use of in silico and in vitro data. In vitro assays have gained traction as a cost-effective and less time-intensive alternative to in vivo assessment. However, in vitro-in vivo correlations are challenging as these tests cannot replicate the physiological microenvironment. Computational approaches help flag toxicity at a structural level, infer cellular response from big data, and integrate heterogeneous data for a systems approach to toxicity. Systems models can simulate exposure-dependent adverse outcomes from in silico/in vitro measurements. Adverse outcome pathways synthesize available knowledge and enable mechanistic interpretation of data, helping devise a reliable screening strategy. An integrated testing strategy that combines all these approaches is the ideal step forward for non-animal approaches to toxicity assessment. Syngene provides in silico and in vitro support in holistic toxicity assessment in addition to animal models. These include the following:
• Data-driven QSAR/RA models for evaluation of toxicity at the molecular level
- Toxicogenomics for evaluating cellular responses at the cellular level
- In vitro assays for safety and new assay development
- System models for providing organ and organism-level response
- AOP development for a knowledge-based, integrated, mechanistic approach to toxicity
About the author








