Overview
Enhanced productivity of target protein is the requirement of any industry, and more so in the biopharma industry, wherein the productivity cost is high. The biopharmaceutical development team at Syngene works with several clients to optimize their productivity and help them reduce their eventual cost of goods. Towards this effort, an intensified fed-batch process has been developed for mammalian cell culture, which results in a 3 to 4 folds yield enhancement over the conventional fed-batch process. The developed technology is for producing monoclonal antibodies and ensures consistent product quality. The products evaluated showed increased productivity in the range of 7-12 g/L.
In this point of view, we discuss how to use technology to enhance productivity using a high seeding density (HSD) approach. The intensified process through converting the traditional fedbatch process to the HSD process could be the answer to increased upstream productivity.
Introduction
The biopharmaceutical industry has seen steady growth over the past three decades. The global therapeutic antibody market was valued at approximately $150 billion in 2019 and is expected to generate more than $300 billion in revenue by 20251 . Currently, there are more than 132 approved biopharmaceutical products in the US and EU, of which 50% are monoclonal antibodies (mAbs), and the remaining products are other biologics2 .
Mammalian cell lines are predominantly used to produce mAbs. The current commercial production method uses fed-batch processes that exhibit productivity titers from 1- 5 g/L. Productivity is measured by the volume of products produced in each batch. In the fed-batch culture, the media and/ or feeds are constantly supplied to the bioreactor during the cultivation, where the desired cells produce the product of interest.
Productivity variation arises depending on the nature of the antibody and the specific cell line which has been used. Higher productivity using the conventional approach is achieved by expanding the manufacturing footprint and requires the addition of larger bioreactors, including facility expansion. An intensified process using perfusion technology is gaining momentum in recent years in this space. This involves constantly removing cell-culture media containing the product from the bioreactor and substituting it with new media.
The upstream process (figure-1) begins with seed expansion after cells are thawed from a cryo-vial through multiple batch-wise passages ranging from mL to L scale. At the end stage of the seed train, known as (N-1), the cells are sufficiently grown for inoculation in the production bioreactor known as N-stage. The use of perfusion at the (N-1) stage for increasing the cell density and using it as HSD – high (initial) seeding density for the fed-batch production culture ( at the N-stage) is a viable approach. HSD cultivation provides higher productivity with stable product quality while having a smaller bioreactor footprint.
Conventional fed batch (CFB)
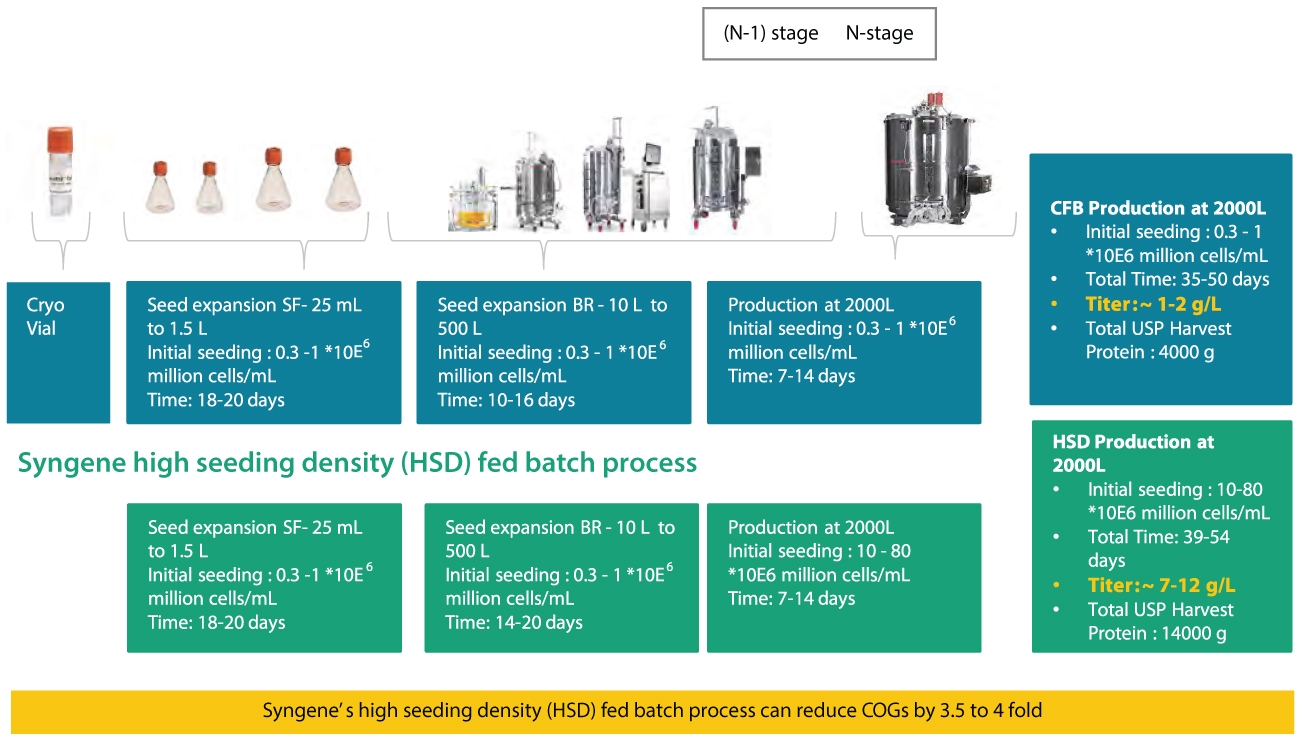
Existing solutions available in the market
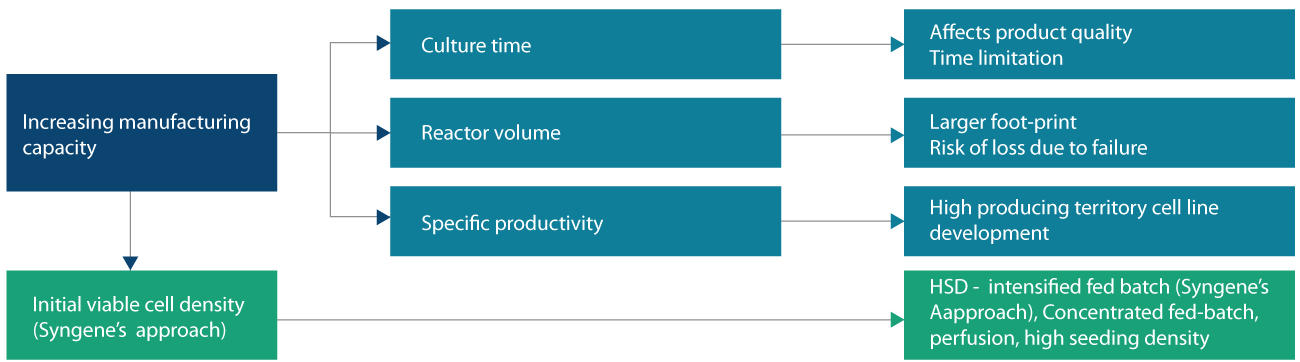
The various methods reported in the literature for enhancing the product titer in the industry are represented in figure 2.
HSD approach
The high seeding density (HSD) approach starts with a high number of productive cells in the bioreactor. It involves conventional fed-batch
with high initial viable cells. Increasing the initial cell density (IVCD) is the best way to improve manufacturing productivity compared to
other conventional approaches.
Perfusion and CFB approach
The perfusion (continuous production) and concentrated fed batch (CFB) (using 50kda filter perfusion)-based approaches at the production stage have more operational complexity in the manufacturing environment compared to the HSD approach. These include the availability of developmental data, longer process runs, and increased risk in case of failure or contamination.
Culture time method
The culture time approach increases industrial capacity but affects the protein post-translational modification (PTM), thereby impacting the product quality. PTM is vital for controlling protein localization, confirmation and stability, and it is critical to ensure the proteins are not affected by metabolic stress.
Reactor volume approach
The reactor volume approach requires capital expenditure and a large manufacturing set-up which remains a challenge for small to mediumsized industries.
Specific productivity approach
Lastly, the specific productivity (Qp) approach ensures high titers but consumes more development time and provides no guarantee that the protein will adapt to the continuous changes in the external environment. The by-products such as lactic acid, ammonia accumulation (figure 3), and other key metabolites negatively impact cell growth and product quality. Hence, they need to be kept in control to achieve higher Qp. The best possible approach is to have higher initial seeding density production by having perfusion at the N-1 stage.
Existing solutions available in the market
In all these approaches, the increase in cell density is limited. There is little supporting evidence to suggest that implementing these strategies as a standalone could significantly increase (> 50 X 106 cells/ml) the viable cell density at the N-1 stage. The standard working cell bank (WCB) also contains low cell numbers, resulting in a longer process time during seed train development. High-density cell banking using the N-1 approach provides respite from these problems and decreases overall process time while achieving higher titers.
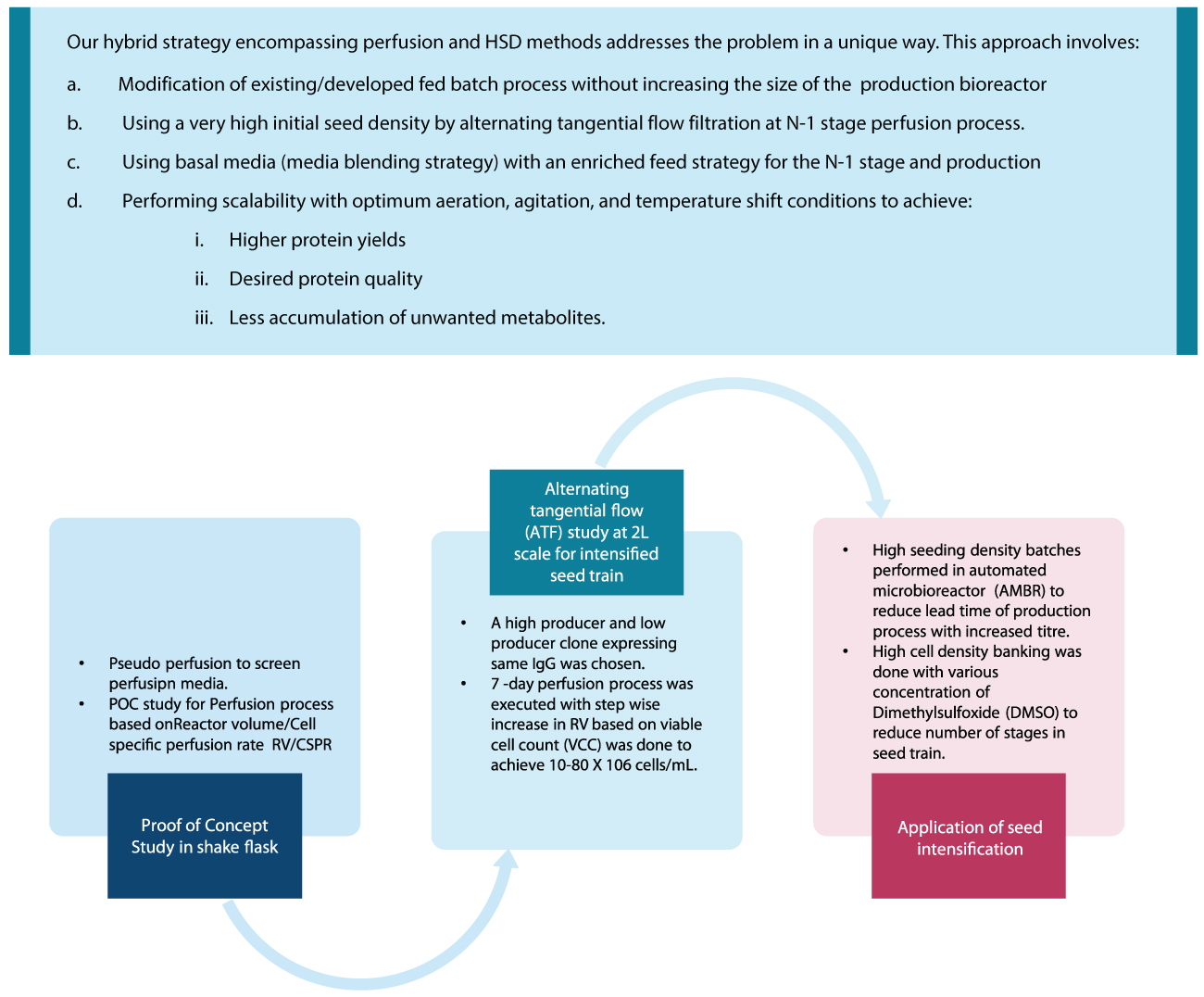
Increased bioreactor efficiency
Generally, cell culture goes through two main phases: growth and protein production phase. The length of the growth phase is determined to a large extent by the proliferation rate of producer cells. When cell density is less, it is recommended to increase the proliferation rate to reach high cell densities. On the other hand, when cell densities reach the higher side, it is recommended to reduce the proliferation rate and enhance protein productivity. In the approaches mentioned earlier, the cell growth phase would take significant processing time. As a result, the bioreactor efficiency period would remain relatively short as the production of therapeutic proteins mainly occurs in the later, less proliferative stage. In order to maintain the bioreactor at its production peak, we can increase initial seed density and reduce the proliferation rate.
We can also channel cell energy from the proliferation stage to the protein production stage, thereby increasing yields. This approach produces higher protein titers in less time than the traditional fed-batch and perfusion process. In addition, we can transform the initial replicative phase (which usually takes more time in conventional fed-batch and perfusion processes) into the production phase, which increases the overall bioreactor efficiency. This is done based on our assessment and understanding of various media and feed combinations and their appropriate modulation based on particular cell lines needs.
Outcomes
Several studies at Syngene have used perfusion cultures at N-1 stages and achieved high cell densities of up to 40 X 10⁶ cells/ml and high biomass in the production bioreactor.
Syngene offers a state-of-the-art facility and technical expertise in a high cell density (N-1) modified fed-batch process with HSD to achieve higher titers of 7-12 g/L. In this approach, you can perform a 3 to 4 folds increase in productivity without any effect on product quality (desired PTMs) compared to the conventional fed-batch method.
Further, this intensified process can be extended to different cell lines, covering all biopharmaceutical molecules (mAbs and complex proteins) available in the market for large-scale manufacturing.
High cell density culture is challenging because of increased oxygen demand and limitations of reactor configuration, accumulation of dissolved CO₂, and other metabolites such as lactate and ammonia. Other metabolites can restrict cell growth and protein production and affect product quality. The proposed intensified approach can keep these challenges in check.
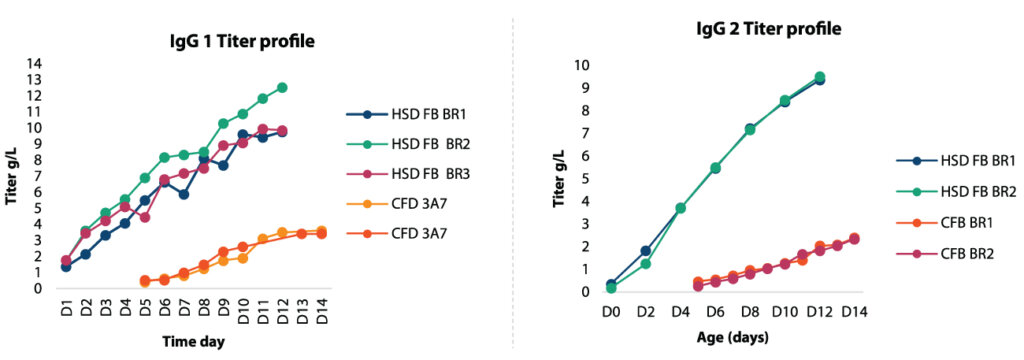
Case studies: IgG1 and IgG2
Studies were conducted to test the developed approach using two different mAbs, and their titer was profiled with time. The graph below compares the conventional fed-batch and the HSD process, where a 3 -4 fold increase in productivity was observed for IgG1 as well as a 3-4 times increase in titer value for IgG2.
Benefits of Syngene’s approach
Significant reduction in Cost of Goods (COGs)
mAbs/biologics production has higher COGs. Recently, annual peak sales per molecule have decreased by 50%, while the average yearly cost has increased by 30%. Under the circumstances, this is an opportune time to introduce new technologies that can give higher productivity without increasing bioreactor volume.
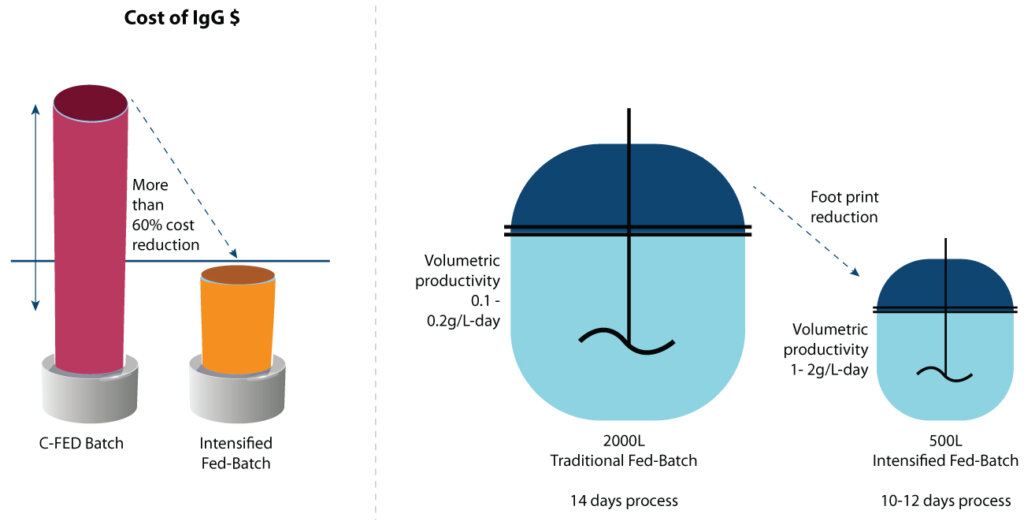
A traditional fed-batch commercial process for mAb usually has a titer of 1 – 3 g/L, running for 12-14 days, translating to volumetric productivity of 0.1 – 0.2 g/L·day. Our studies have shown that the intensified process has volumetric productivity of 1 – 2 g/L·day. Increasing volumetric productivity enables a reduction in bioreactor size and manufacturing space for upstream biomanufacturing and improves production efficiency while lowering COGs (figure 5).
In the intensified approach, the cell metabolism remains unchanged as in the conventional approach. This results in consistent, specific productivity and desired PTMs. We have also assessed various product quality attributes such as size, charge, glycosylation in a comparative mode for a Mab product, produced through regular fed batch process or using HSD fed batch.
Conclusion
The intensified fed-batch process is a viable alternative for higher protein production at a large scale without expanding the current manufacturing footprint. A media blending strategy developed at Syngene keeps the culture viable for a longer time and minimizes undesired waste accumulation during the production process. With this hybrid method, you can achieve higher titers of 7-12 g/L (a 3-to-4- fold increase) without impacting product quality. By adopting Syngene’s intensified N-1 and HSD production, overall COGs can be reduced with fewer batches (COGs include all the direct costs involved in manufacturing products, including the cost of labor, raw materials, and overhead costs associated with running a production facility). You can also minimize space-time requirements without revamping the existing manufacturing footprint.
About the author








