Role of Long Acting injectables in Drug Delivery
Long-acting injectable (LAI) is a revolutionary drug delivery system that addresses some of the most persistent challenges in treating chronic diseases. These include patient non-compliance in maintaining medication regimen, fluctuating drug levels in the plasma, need for frequent dosing, and more. By delivering therapeutic agents over extended periods, LAIs can significantly improve patient adherence, reduce adverse events, ensure targeted delivery, and enhance the quality of life.
This article delves into the various types of LAIs, technologies used, challenges in developing LAIs, and how leading CRO CDMO Syngene supports clients in bringing this delivery mode to patients efficiently and effectively.
Transforming therapy with long acting injectables
One of the most significant advantages of LAIs is their ability to enhance patient adherence to treatment. Treating chronic diseases often requires daily oral dosing, which can be burdensome and lead to poor adherence. LAIs, with their ability to deliver the drug over an extended period (e.g., from weeks to months), reduce the treatment burden on patients. LAIs also provide a steady state of drug concentration in the plasma, minimizing peaks and troughs associated with daily dosing. This steady state is beneficial for maintaining long-lasting therapeutic effects and reducing the risk of adverse events.
By maintaining constant drug levels in the therapeutic window. LAIs also help in mitigating the potential risk of drug resistance and relapse, particularly in conditions like cancer, psychiatric disorders, HIV/AIDS, and tuberculosis. LAIs are being utilized in the treatment of several diseases, including cancer, malaria, tuberculosis, diabetes mellitus, psychiatric disorders, eye disorders (e.g., age-related macular degeneration), HIV/AIDS, and contraceptive applications. Their ability to provide targeted delivery and maintain steady plasma levels makes them ideal for these conditions.
Types of long acting injectables and technologies used
1. Systemic-acting injectables:
- Oil-based injectable solutions: These solutions provide a slow-release profile by suspending the active pharmaceutical ingredient (API) in an oil-based carrier.
- Injectable drug suspensions: These suspensions contain micronized or nanonized particles of drugs in a liquid medium, offering controlled drug release over time.
- Polymer-based implants and in-situ forming implants: These implants are made of biocompatible polymers that slowly degrade, releasing the drug at a steady rate.
- Polymer-based microparticles/microspheres: These particles provide controlled and sustained release of drugs through the gradual breakdown of polymers.
2. Local acting injectables:
- Polymer-based and lipid-based microparticles: Used for localized delivery, these microparticles offer targeted release at the site of action, minimizing systemic exposure.
- Intrauterine devices (IUD) and intravaginal rings: These devices are particularly useful in contraceptive applications, releasing hormones over extended periods.
Major challenges in developing Long Acting injectables
Complex formulation development
- Q1/Q2/Q3 criteria: Most of the LAI-based drug products need to demonstrate the Q1/Q2/Q3 sameness criteria with the generic product in terms of qualitative, quantitative, and physiochemical properties/performance criteria — making it extremely challenging to formulate any LAI product.
- Controlled release mechanisms: Achieving a precise and predictable drug release profile over extended periods is challenging. The formulation must balance immediate and controlled release to maintain therapeutic levels.
- Polymer and excipient selection: The choice of polymers, lipids, or other excipients that make up the matrix of LAIs is critical. These materials must be biocompatible, biodegradable, and capable of providing the desired release kinetics without causing adverse reactions. Multiple critical material attributes (CMA) must be considered when selecting these rate-controlling polymers.
- Drug-polymer compatibility: Not all drugs are compatible with the polymers or carriers used in LAIs. Drug-polymer interactions can affect the formulation’s stability, release profile, and overall efficacy.
- Complex characterization techniques: High-end equipment and seasoned expertise are required to characterize the LAIs.
Pharmacokinetic (PK) and Pharmacodynamic (PD) correlation
- Altered PK/PD profiles: LAIs often exhibit non-linear pharmacokinetics due to depot formation at the site of injection, variable drug release rates, and differences in absorption. Establishing optimal dosing regimens to ensure efficacy without toxicity is complex.
- In vitro-in vivo correlation (IVIVC): Developing a robust IVIVC is essential to predict in-vivo performance based on in-vitro data. However, due to the complexities of in-vivo drug release mechanisms, achieving meaningful IVIVC can be difficult and requires comprehensive in-vivo studies.
- In silico modeling – As the dosing frequency of LAI is very high (monthly), the formulator must use advanced in silico modeling to establish the in vitro-in vivo correlation (IVIVC).
Manufacturing and scalability
- Scale-up from lab to commercial production: Scaling up from laboratory or pilot-scale batches to full-scale commercial production can introduce new challenges. The methods and equipment used at different scales can affect the product’s properties and performance. As the manufacturing of LAIs involves a complex multistep process, the scale-up or technology transfer from lab-scale to commercial GMP scale demands a parametric approach, with a dedicated focus on control strategy for critical process parameters (CPPs).
- Batch-to-batch consistency: Ensuring consistent quality, potency, and release profiles across different manufacturing batches is a significant challenge. Minor variations in raw materials, process parameters, or environmental conditions can affect the final product quality. The batch-to-batch reproducibility of LAI is a concern in terms of achieving the desired yield and constantly ensuring that the final product meets the defined critical quality attributes (CQAs).
In vivo study requirements
- Extended in vivo studies: Given that LAIs are designed to act over weeks or months, in-vivo studies to assess pharmacokinetics, pharmacodynamics, and safety must also extend over long periods. This requirement increases the time, cost, and complexity of development.
- Population extrapolation: Ensuring that the LAI is effective across diverse patient populations (varying in age, weight, comorbidities, etc.) can be challenging, requiring careful design of clinical trials to account for variability
Regulatory challenges
- Regulatory requirements for novel delivery systems: Developing a novel LAI often requires extensive data on safety, efficacy, stability, and manufacturing consistency. The regulatory path can be longer and more complex than traditional oral or simple injectable formulations.
- Establishing Q1/Q2/Q3 sameness: For generic LAIs, demonstrating Q1/Q2/Q3 sameness to the reference product is challenging due to the complex dosage form, longer release time, and formulation/process factors that affect drug release, absorption, and distribution.
Syngene’s capability in developing high-quality LAIs
Application of machine learning in LAIs
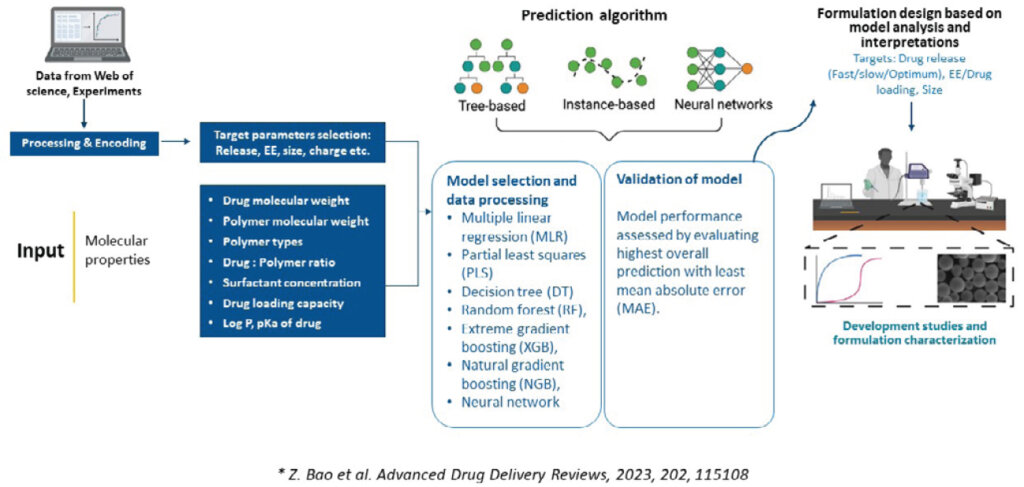
LAIs involve complex processes that require precise optimization of various parameters such as drug release rates, particle size, and encapsulation efficiency. Machine learning (ML) has emerged as a valuable tool in this domain, allowing researchers to predict the behavior of different formulations based on molecular properties and processing parameters.
At Syngene, we use ML techniques like multiple linear regression models, decision trees, and neural networks to optimize formulation design. We also use ML for in vivo prediction since the frequency of LAI dosing is usually spread across several months, making in vivo study durations too high to accommodate multiple studies. This approach greatly reduces the need for extensive in vivo studies
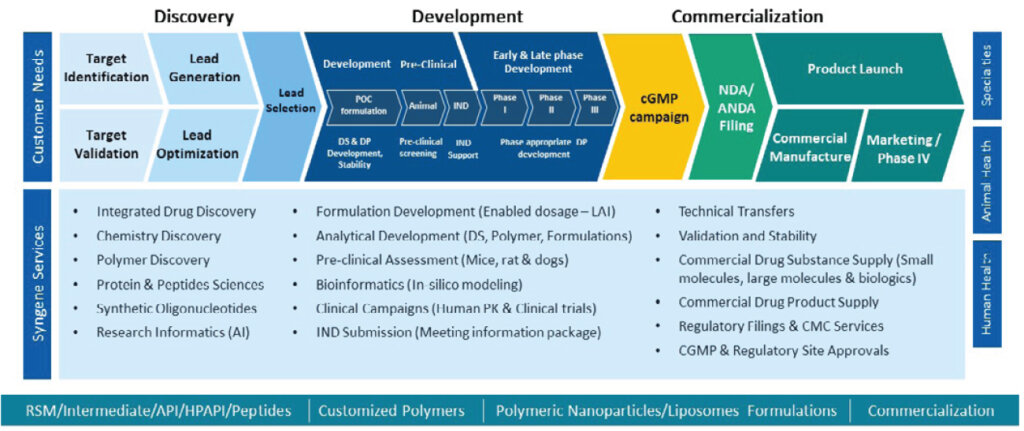
Integrated development of LAIs: Early discovery to commercial
Developing a long-acting injectable product involves a multi-stage process from drug discovery to commercialization. We have significant experience in integrated drug development of LAIs using our proprietary SynVent platform. SynVent integrates synthetic and medicinal chemistry, biology, DMPK, and toxicology while leveraging our extensive drug discovery knowledge and project management proficiency. This approach lets clients concentrate on essential milestones and their core scientific requirements while we progress their molecule from early discovery to commercial – efficiently and cost-effectively.
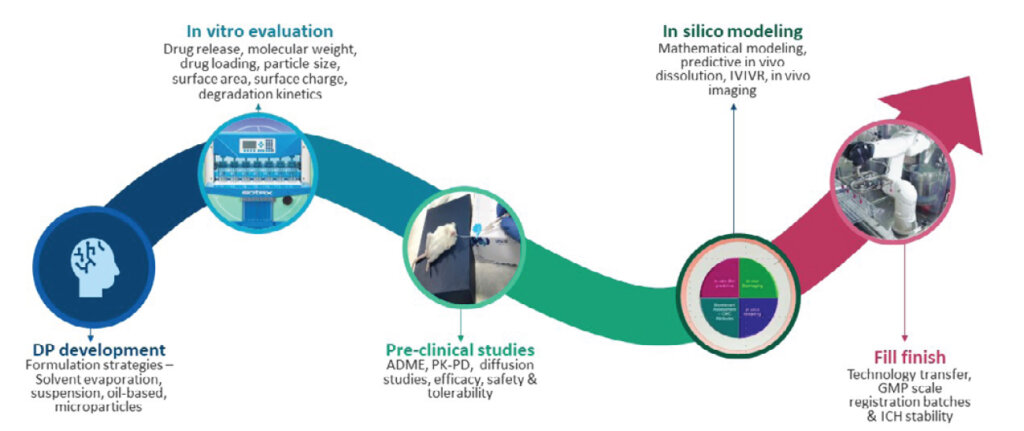
Lab to GMP-scale development
We have extensive experience in progressing LAIs from lab to GMP scale-up. Apart from expertise in formulation strategies, in vitro evaluation, complex characterization, and preclinical studies, we offer in silico tools for mathematical modeling, predictive in vivo release, in vitro in vivo correlation (IVIVC), and in vivo imaging. We also undertake final fill-finish via our state-of-the-art, high-speed facility, which has a capacity of 1 million vials/day. Our first-time-right technology transfer capability from lab to commercial ensures efficient use of time and resources and faster time-to-market for clients.
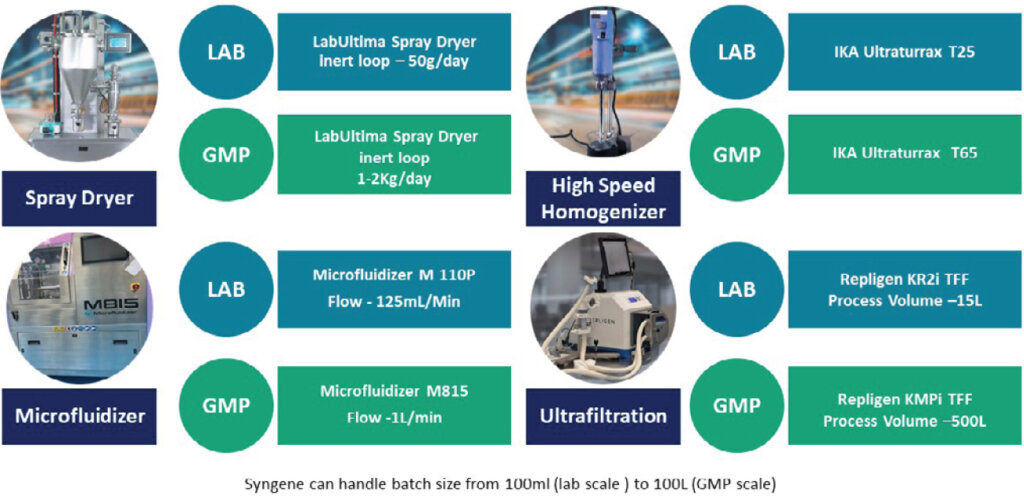
Our LAI formulation capability
Our facilities are equipped with the latest tools and technologies to develop LAI formulations. These include spray dryers, microfluidizers, highspeed homogenizers, and ultrafiltration equipment. Our facilities can handle batch sizes from 100mL (lab scale) to 100L (GMP scale).
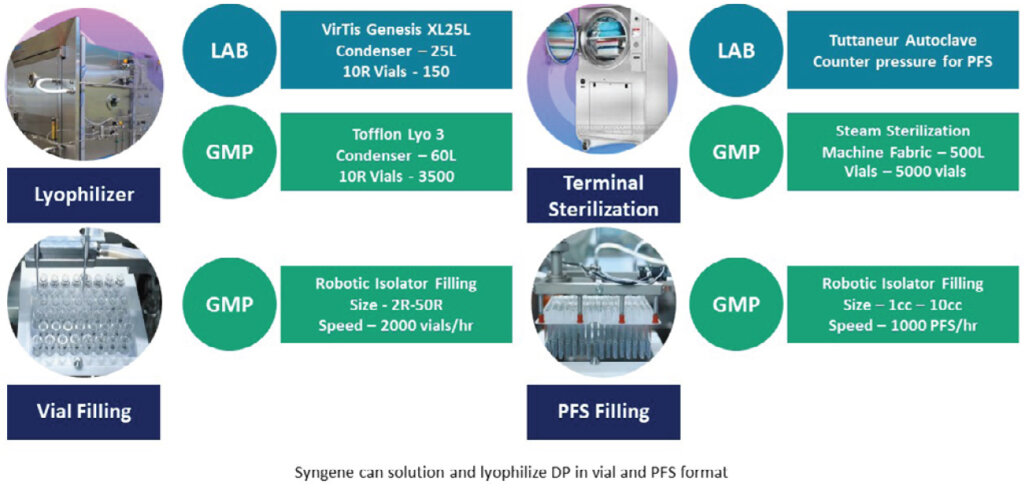
We have a state-of-the-art facility to undertake fill-finish of solutions and lyophilized drug products. The sterile fill-finish facility has an isolator based filling (with robotic arm) line with a total annual capacity of 5 million vials/year and 2 million pre-filled syringes (PFS)/year. The facility includes various container closure system configurations, including vials and PFS, with the capability to carry out lyophilization and terminal sterilization, ensuring sterility of drug products.
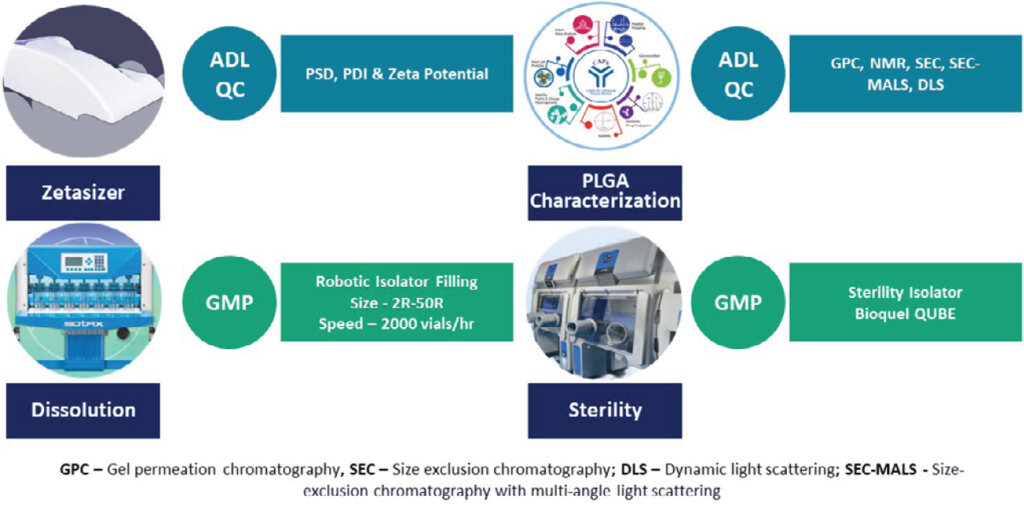
Syngene’s Analytical Expertise for Long Acting injectables
With more than 30 years of experience in analytical development, we have significant expertise in method development, method validation, method transfer, and application of analytical expertise to ensure the delivery of quality drug products for early and late-phase drug development programs. We have expertise in developing phase-appropriate methods, validation, and transfer of the methods for drug substances and drug products for simple and complex, long-acting parenteral formulations. We analyze small molecules (RSMs, intermediates, and APIs), semilarge molecules (peptides and oligonucleotides), PLGA polymer, and large molecules using a variety of spectroscopic, chromatographic, and physiochemical techniques for high-end characterization as per regulatory standards resulting in higher success rates at the clinic and beyond. Further, the team has significant experience in conducting reverse engineering and Q1/Q2/Q3 characterization for LAIs.
Case Study on Developing a Stable Lipid Injectable Nano-Emulsion for IV Administration
The client wanted to develop a non-Q1/Q2 nano-emulsion for IV administration. The requirement was for a physiochemically stable formulation with particle size distribution (PSD) of NMT 100 nm and the zeta potential of NLT 20 mVstable during storage. The project was challenging because of the need to ensure emulsion stability/prevention of crystal growth formation, size reduction, and homogenization, as well as maintaining uniform globule size distribution with zero to minimum lipid degradation. Our team developed a robust manufacturing process to ensure the desired output. By meticulously controlling aqueous and non-aqueous phases, employing stabilizers, and defining critical process parameters, we ensured uniform droplet size and stability. Our adherence to stringent quality standards, including globule size measurements as per USP <729> for lipid injectable emulsions. The team’s dedication and meticulous planning resulted in successfully developing a non-Q1/Q2 nano-emulsion suitable for commercialization
Case Study on Developing and Optimizing IV Lyophilized Liposome Formulations
The requirement was the development and optimization of an IV lyophilized liposome formulation. The target product profile included PSD
(NMT 100 nm), capability for high drug loading, ease of reconstitution, and a scalable and reproducible process.
The project posed several challenges. This included maintaining an appropriate lipids-to-membrane rigidifier ratio, PSD and drug loading for
liposomes, a robust lyophilization cycle, drug release, and product performance after reconstitution.
We developed liposomes using spray drying technology as an efficient alternative to traditional methods. To overcome the challenges of PSD
and lipid stability, our multidisciplinary team collaborated to optimize spray drying parameters and refine the formulation process. Through
systematic composition optimization and process refinement, we successfully generated unilamellar liposomes, showcasing a scalable and
efficient manufacturing process to the client.
Unlocking the Full Potential of Long Acting injectables
Developing long-acting injectables is a multifaceted process that involves overcoming significant scientific, technological, and regulatory challenges. However, the potential benefits—such as improved patient adherence, optimized drug delivery, and better management of chronic diseases—make them a crucial area of focus in modern pharmaceutical development. Continued advancements in formulation science, predictive modeling, and manufacturing technologies are key to addressing these challenges and unlocking the full potential of LAIs. By partnering with CRDMOs such as Syngene and their experienced team of scientists, CMC expertise, and advanced technology, biopharma companies can greatly ease the process of bringing LAIs to market faster.
About the author








