Introduction
The Healthcare sector was never in greater focus than in 2020. With the pandemic continuing unabated, the world looked to Science and the scientific community for deliverance. And Science did not disappoint. In less than a year of the pandemic, thanks to concerted global efforts, more than 150 vaccine candidates became available as a possible cure for COVID-19.
While 2020 was all about discovering a vaccine, 2021 is going to be about how to take the vaccines to market in the shortest possible time. With more than 2.6 million deaths worldwide (figures as on 9 March, 2021) and counting, the need for speed cannot underscored.
However, to decipher the manufacturing challenge, one needs to understand what goes behind producing a vaccine — raw materials, technology, and a complex manufacturing process. And finally, how and why Contract Manufacturing Organizations (CMOs)/ Contract Development and Manufacturing Organizations (CDMOs) are better positioned to bridge the gap between vaccine demand and supply, safely and at speed.
Types of vaccines
Vaccines are generally classified as follows:
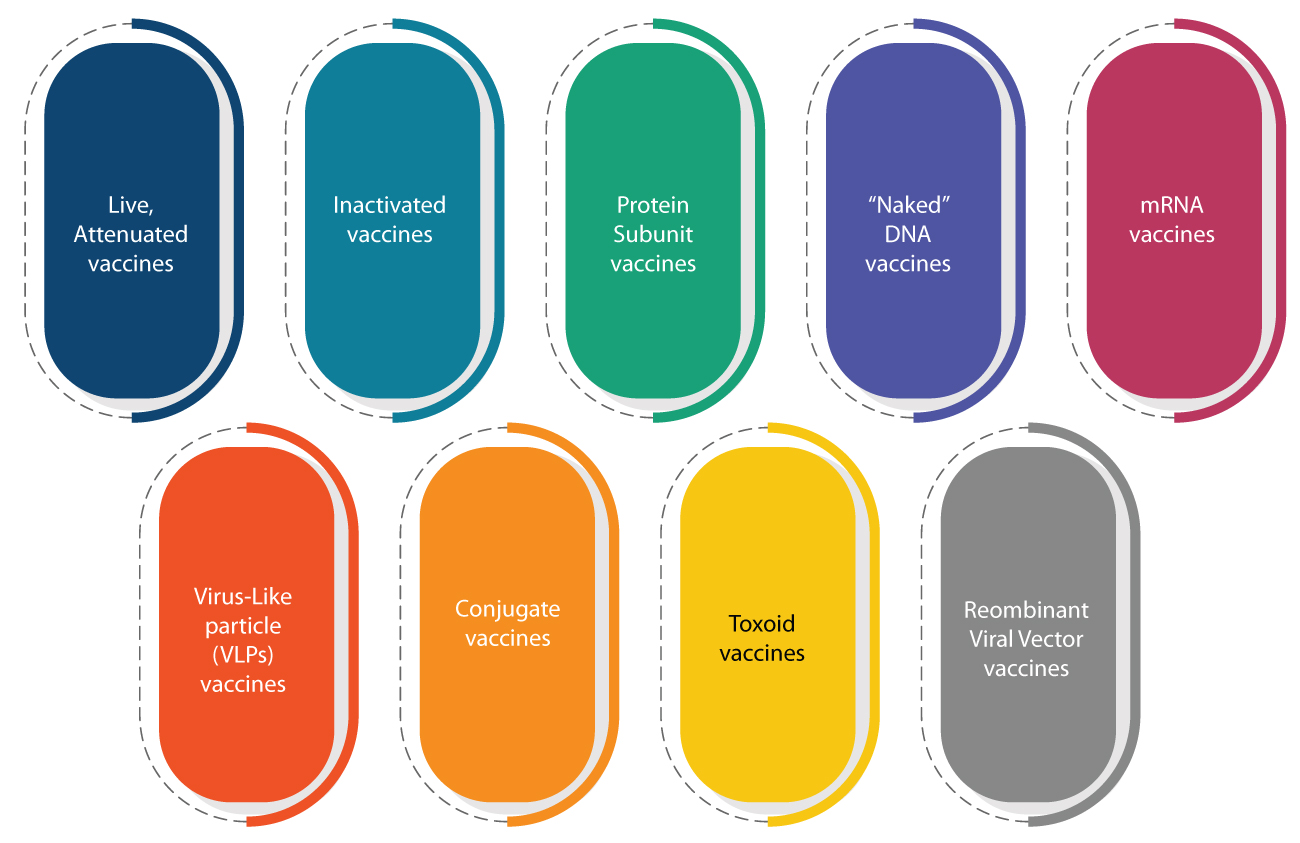
COVID-19 vaccines being developed, currently, are based on these platforms.
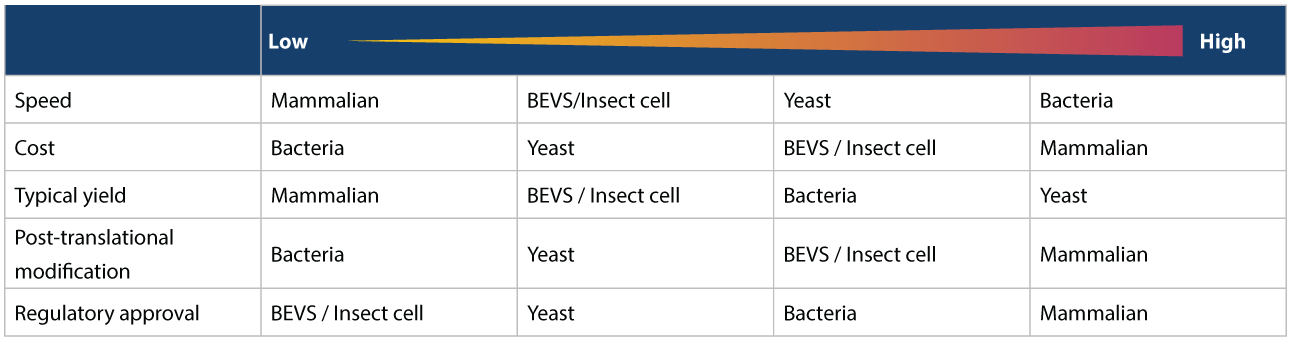
Various Expression Systems and Hosts are used to produce the vaccine candidate. As shown in the image below, we can consider no single expression system an overall “best option” since each one has certain advantages and disadvantages. Also, not all expression systems can be used to produce all antigens. Hence, the options available also depend on the vaccine to be manufactured.
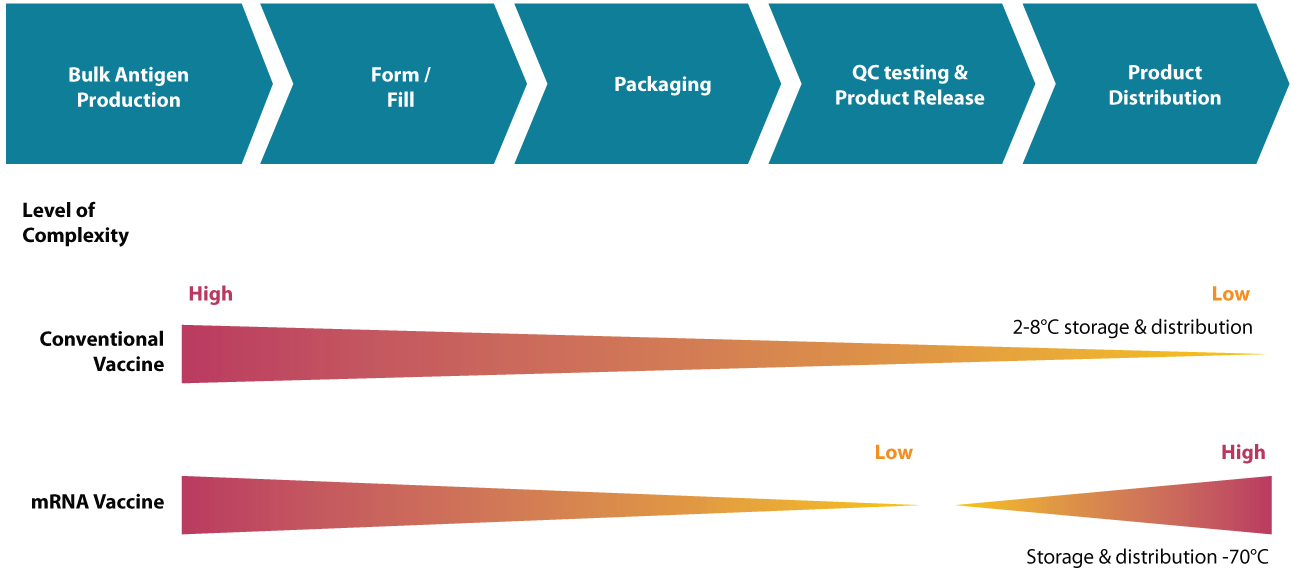
Vaccine manufacturing process
The key steps in manufacturing vaccines are as follows:
Challenges in manufacturing vaccines
While COVID-19 vaccines surpassed all expectations by becoming available to the general population in less than a year, this is an exception rather than the norm. A typical vaccine usually takes 10 to 12 years to reach the market after discovery efforts begin. With most pharma and biotech companies using conventional facilities for manufacturing vaccines, this leads to further delays.
Limitations of conventional facilities
Convention facilities have limited flexibility and are capacity constrained. Hence, they cannot handle a sudden spike in demand — as necessitated during a pandemic or an outbreak such as COVID-19. Commercial manufacturing facilities are also dedicated to a specific vaccine product, and do not have the flexibility to switch to producing large volumes of a new vaccine. This is especially challenging because interrupting vaccine production can lead to the failure of national immunization programs that deliver mandatory vaccines for Expanded Programme on Immunization (EPI). High upfront investment during product development is also deemed risky, given the uncertainty of Phase-III outcomes and demand, postlaunch.
Cost implications of building new capacities
For biotech and pharma companies to build new capacities, it would be a risky proposition, given the huge capital expenditure involved. The cost of setting up a new vaccine manufacturing facility with supporting functions and having a capacity for 100 million doses is estimated to be around $60-80 million. Supporting operations would include Utilities, Quality control (QC), Quality Assurance (QA), Warehouse, and effluent treatment systems for environment clearance. Further, it would take approximately 3-5 years for the facility to be fully operational.
Some other challenges include:
High-quality equipment with automation controls that need time and coordination for interfacing and setting up systems.
Need to re-test high integrity equipment, utilities, and facilities with environmental monitoring programs.
Significant level of checks and controls prior to release.
High level of qualifications to ensure equipment and facilities are suitable for use and commissioning testing for utilities and equipment.
Inability to perform final manufacturing until the facility is ready.
Time and cost of setting up a manufacturing facility for vaccines is 2–3 years, with the cost of setting up a facility exceeding $50 million. Even in an emergency, timelines might be shortened by 1.5 to 2 times as compared to normal timelines.
Regulatory challenges:
Vaccines are one of the most regulated materials in the pharma and biotech space. Historically, vaccines have been subjected to limited characterization, and hence robust regulation and oversight have been imposed to ensure safety and efficacy.
Even after a Biopharma company has completed the manufacturing process with proven clinical studies, and submitted the data to the regulatory authorities, the process has been mandated to be followed.
The final vaccine needs to be released to confirm that any change to the process or equipment has not impacted the quality of the vaccine. This requires extensive testing and documentation.
This process adds significant time and cost to the delivery of vaccines.
Distribution challenges:
Distribution challenges involve logistic and cold chain requirements to ensure vaccine safety. A single vaccine may need different storage conditions and safety until it is administered. Some examples of sensitivity include:
Most pediatric vaccines like oral Polio vaccine (OPV), Measles, Mumps, and Rubella (MMR), HPV and Influenza vaccines.
Live vaccines like Bacillus Calmette-Guerin (BCG) for tuberculosis & MMR.
Live vaccine (Bacterial or viral) are prone to potency loss when exposed to extreme temperatures.
Non-live vaccines are more stable and hence have reduced requirements for temperature.
The finished product of vaccines must also be stored at 2–8°C which is the vaccine “cold” chain, as defined by the World Health Organization (WHO). It has been observed that COVID-19 vaccines have a higher sensitivity, which poses a huge distribution challenge.
Advantage CMOs/CDMOs
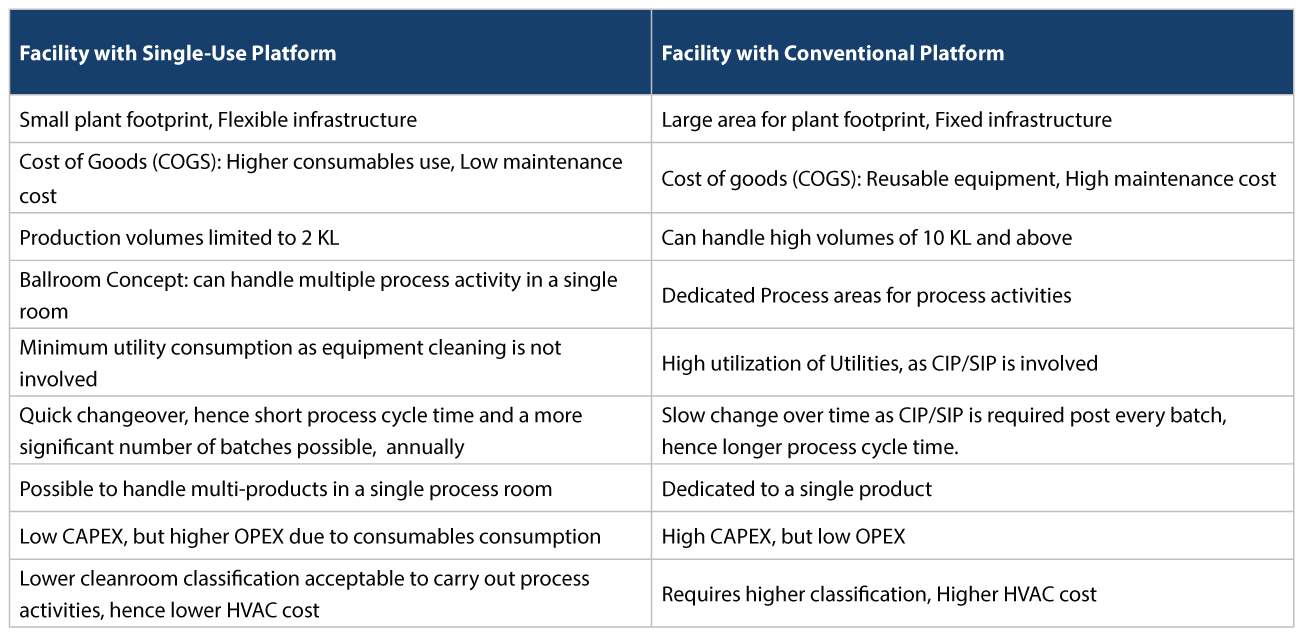
Easing capacity constraints In such a scenario, using CMOs/CDMOs to take on vaccine manufacturing provides significant risk mitigation. For starters, they could ease capacity constraints, and help in responding more quickly to changes in demand. For example, today, various vaccine approaches being taken to address COVID-19, rely on r-proteins, viral vectors, or mRNA. Many CDMOs already have the expertise in this area to produce firstgeneration vaccines, at speed. Accelerating regulatory approvals Vaccine manufacturers can also accelerate regulatory approvals if regulators have previously authorized similar products before. For example, several platform technologies being used to produce COVID-19 candidate vaccines are relatively untested. The resulting vaccines’ modular nature could mean, that regulatory approval can be expedited, as these technologies become more common place. Flexible Facilities Most CMOs/CDMOs also use flexible facilities with single-use disposables for manufacturing, which has several advantages. This is as opposed to reusable systems used by traditional Biopharma companies who usually have conventional facilities. The conventional facilities use stainless steel for upstream and downstream activities which have several disadvantages that need to be addressed (Refer Tables 1, 2, and 3 below).
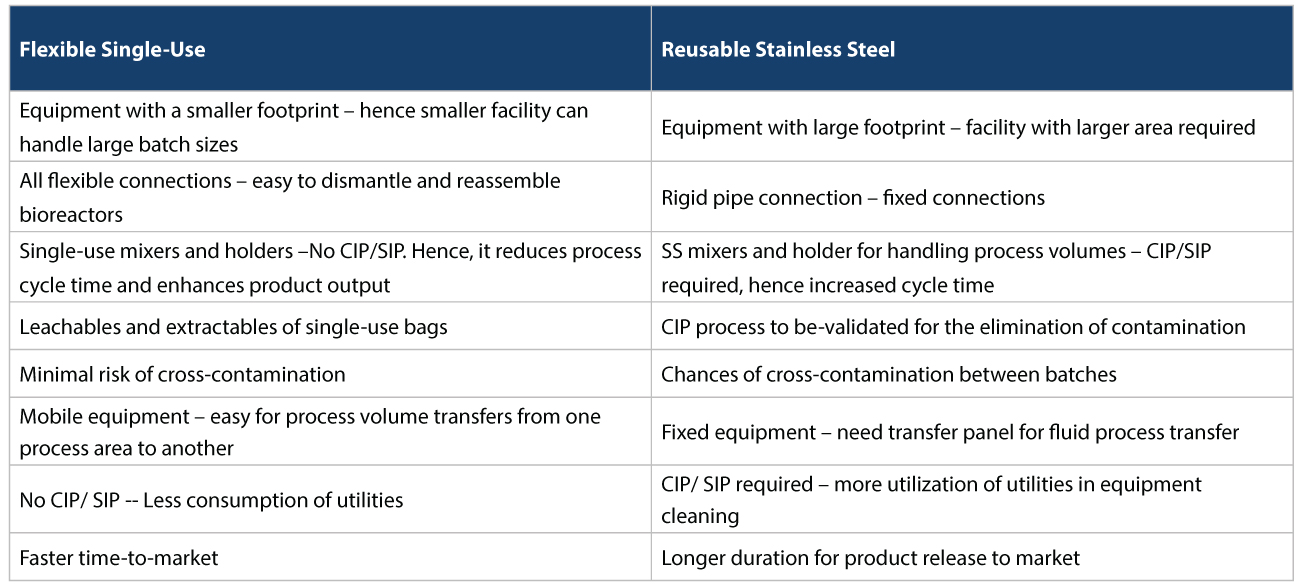
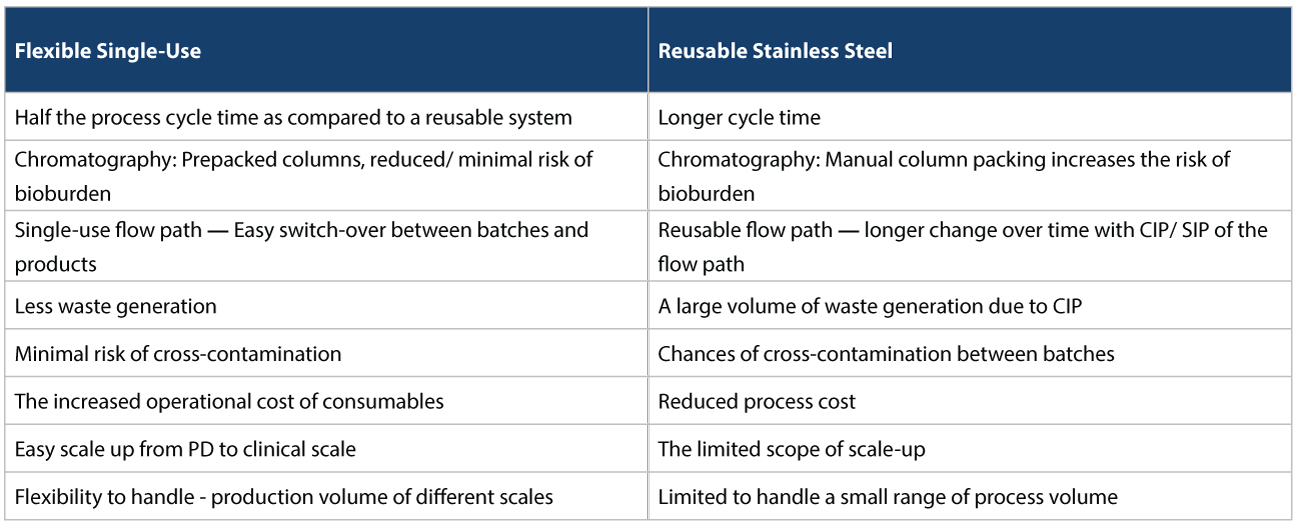
Distribution capability
CMOs/CDMOs are better equipped from a distribution perspective as well. They have access to well-organized, cold chain transport systems comprising Envirotainer, cold box, and refrigerated reefer for temperature-sensitive products. These ensure, product safety and efficacy of the vaccines are maintained until delivery
Syngene’s capability and capacity in vaccine manufacturing
CMOs/CDMOs like Syngene can offer end-to-end services across the manufacturing value chain. Our cGMP-complaint facilities are equipped with flexible, single-use systems for both upstream and downstream activities, and provide advantages of space, time, cost, and compliance.
Mammalian manufacturing:
For its disposable-based mammalian facility for clinical and commercial manufacturing supplies, Syngene has 4 x 2,000L Single-Use bioreactors to support cell expansion 100 500 2,000L. It has 3 x 2KL bioreactors currently in operation. While two Upstream suites allow parallel processes, Harvest is by centrifuge or depth filtration, and downstream comes with post-viral segregation. Upstream manufacturing: Our Upstream capabilities include two suites (from Inoculum to Production reactors), 3 x 2KL
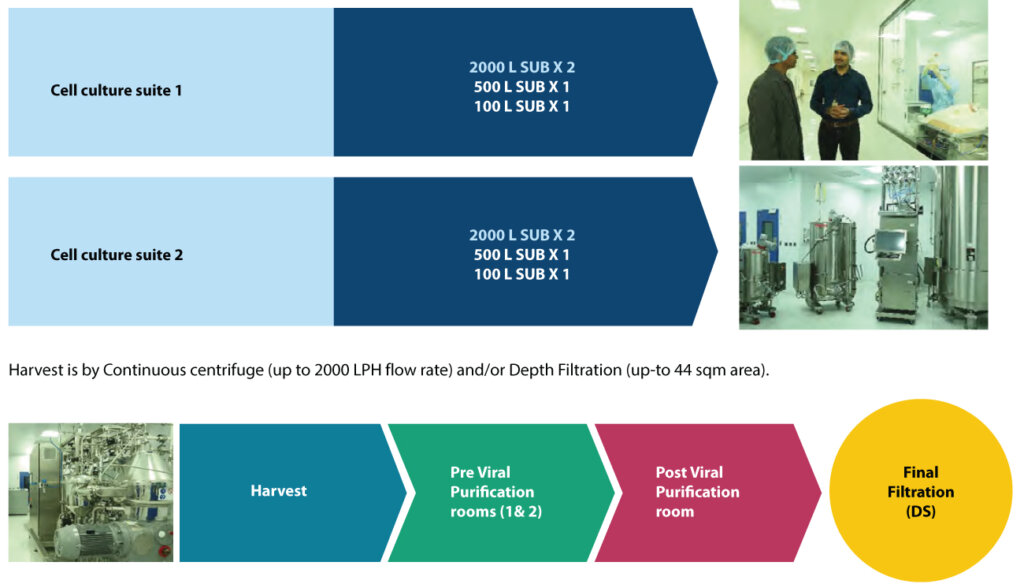
Our Downstream capabilities include:
• Pre-viral (two rooms – with all chromatography steps, UFDF, Viral filtration)
• Post viral (one room – UFDF and final filtration – closed operation),
• Can handle products with 4 chromatography steps – Flow rate up to 2000LPH
• Column size > 800mm (depends process and operating conditions), currently till 600mm
Microbial manufacturing:
Our cGMP facility for microbial manufacturing can produce recombinant proteins from bacteria and yeast cell with intracellular or secreted expression. Critical equipment includes 200L (SS) and 500L (SS) fermenters, Continuous centrifuge, Cell homogenizer, Chromatography systems and TFF systems. The facility can take up manufacturing scale of 500L (SS) fermentation upstream, continuous centrifuge of 200LPH, Homogenization of 300LPH, and downstream process using 1000L refolding single-use system. It has 600 to 2000LPH Chromatography system with associated columns up to 60cm, 10 sqm tangential flow filtration, and final filtration of drug substances.
Syngene’s Capability and Capacity in newer vaccine platforms
We have the capability and capacity to produce 5–200 million doses of equivalent drug substance across Protein Sub-units, r-BEVS, mRNA, and Viral Vector vaccine platforms. These are the new approaches currently being used for manufacturing vaccines for COVID-19. The manufacturing facilities comply with the biosafety requirement of BSL-1 and BSL-2, which provide a broader opportunity in manufacturing vaccines across different platforms.
Conclusion
COVID-19 has taught the world the value of scientific collaboration in discovering, producing, and distributing a vaccine, quickly. Technology has also played a significant role, especially when it comes to mRNA vaccines being developed by Moderna and Pfizer. These could well lead the way in combatting other deadly viruses in the future. However, to avoid high rate of fatalities as with COVID-19, manufacturing processes and technologies must evolve. Speed of response from threat identification, testing protocol implementation to vaccine deployment, needs to increase. Flexibility in manufacturing a wide range of vaccines should also be available. Currently, CMO/CDMOs like Syngene are uniquely positioned to meet this challenge. Years of collaborating with clients has helped them become knowledge centers in themselves, besides being technologically ready to take up new challenges. Biotech and pharma companies need to take advantage of CMOs/CDMOs’ superior knowledge and expertise, and partner with them for manufacturing vaccines in the shortest possible time.
About the authors









