Introduction
Drug development, from the initial discovery of a promising target to commercial launch, is an expensive, lengthy, and high-risk affair with poor success in the clinics. It is an even bigger struggle for emerging pharma companies (often the innovative engines for many of today’s novel products), given their lack of resources and facilities in an increasingly competitive market.
With a growing focus on early development to avoid late-stage attrition, contract development and manufacturing organizations (CDMOs) a r e increasingly becoming critical components to help overcome these challenges. CDMOs with end-to-end expertise can help pharma companies develop strategies to achieve clinical success while ensuring time and cost efficiencies.
This article discusses the key strategies adopted by leading CDMO Syngene to accelerate the lead to first-in-human (FIH) clinical trial journey of small molecules for clients.
Challenges in the path leading to FIH clinical trials
Successful execution of the product journey, from the earliest stages of development to FIH trials, requires thorough planning and seamless integration. Some of the common challenges involved in the early development process to reach FIH trial are listed below:
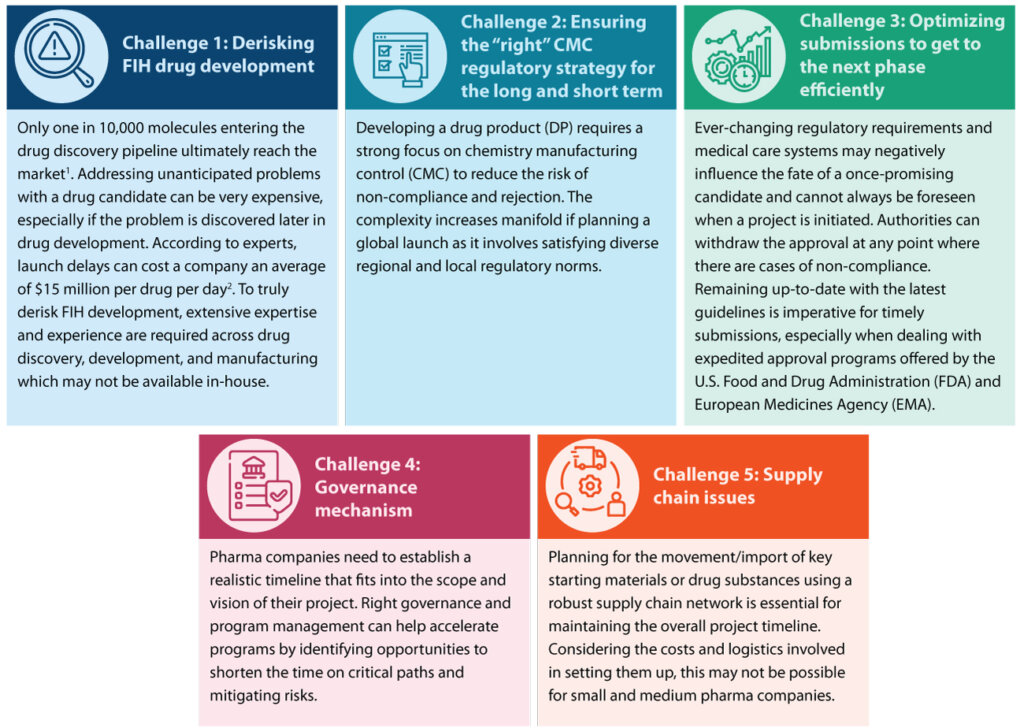
Syngene’s approach to successful FIH clinical trials
At Syngene, the overall FIH strategy involves categorizing drug development into preclinical, development, and clinical stages and integrating them. The first step is thoroughly understanding the intrinsic physicochemical and biopharmaceutical properties to anticipate the developmental challenges. Developability classification system (DCS) assessment is a preliminary step to assess dissolution/solubility/permeability-limited bioavailability. Accordingly, the development requirement for conventional or enabling technology is assessed at the lead optimization stage. Other important considerations include screening of salt and polymorphs and establishing controls in drug substance (DS) process development. We also ensure the development of toxicological formulations keeping FIH in mind, to minimize the need for bridging studies later. To ensure the success of your CMC regulatory strategy, we categorize the FIH journey into six stages (Figure 2). At the end of each phase, the work is reviewed by subject matter experts (SMEs) to see whether the project is ready to move to the next phase or not. The stage gate concept helps us make the right decision, weighing the risks and benefits at each stage, increasing the chances of success at the clinic.

Critical data required for derisking drug development
Derisking drug development strategies typically focus on accumulating as much knowledge about a compound as possible during the discovery and preclinical testing stages to reduce the chances of late-stage attrition. For this, we identify all potential risks to do with safety, efficacy, and synthesis in the early stages of discovery.
Some of the important product attributes that we use to help derisk the development process are as follows:
- Critical attributes such as dissociation constant (pKa), log p/D, solubility, and human colon carcinoma 2 (Caco-2) with 10 mg of the compound at the discovery stage to understand drug developability
- Microscopy assessment (PSD and birefringent pattern) for the solid state to know the solid phase and impact on exposures solubility vs. permeability limited bioavailability by dosing solution vs. suspension formulations
- Brick dust or greasy classification based on the log p and melting point enables formulation (amorphous solid dispersions [ASDs] or lipid-based) decision
- Pharmacokinetic dose range finding studies for dose margins
Syngene’s four levers to accelerate FIH programs
We believe that four levers can have a significant impact on accelerating FIH programs (Figure 3)
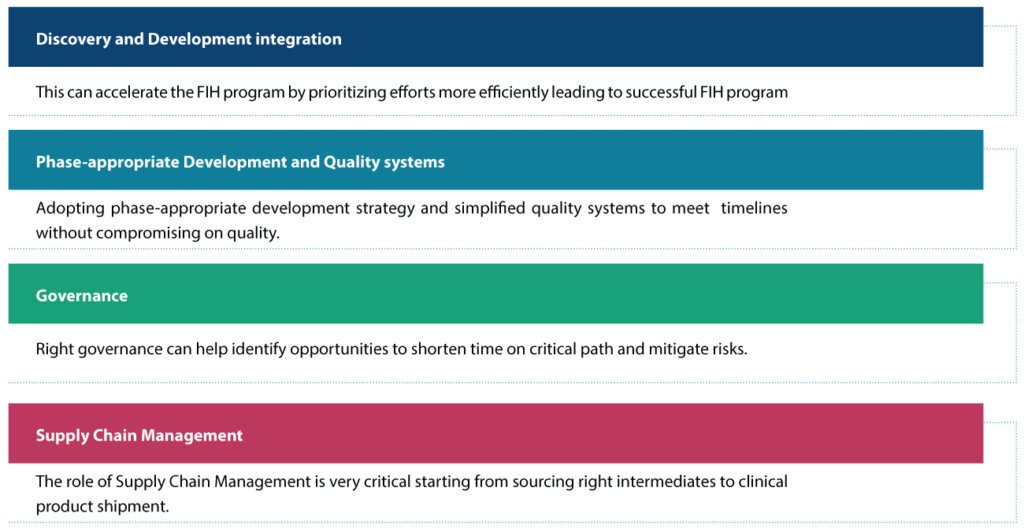
Discovery and Development integration
A fully integrated discovery and development continuum enables the invention and selection of the highest-quality drug candidates. Key decisions taken in the discovery phase strongly influence the overall development cost, time, and, ultimately, the probability of delivering successful new medicines.
Discovery and development scientists should work closely at an early stage to identify development risks and have a strategy to mitigate the risks. Product design attributes that Syngene considers at the discovery to development interface include:
- Target product profile and concurrence
- Critical quality attributes and design specifications
- Technical issues and risk assessment
- Safety assessment considerations
- Environmental, health, and safety considerations
Phase-appropriate development and quality systems
Adopting phase-appropriate development strategy and simplified quality systems are important factors to be considered to meet the timeline without compromising quality. At Syngene, the following important points are considered while employing a development strategy at each phase:
- The simplest possible formulation that is consistent with the clinical goals of early-phase safety and proof of concept studies
- Phase-appropriate analytical approach for verification or validation and involving regulatory and quality persons early in the game
- Parallel development activities for DS and DP (to enable rapid material and knowledge transfer)
- Simplifying the procedure to ensure speedy delivery of clinical material with appropriate quality control systems
- Due diligence for genotoxic impurities
- Physical form and biopharmaceutics assessment before the tox stage
- Assessing technology requirements before the tox stage
- Exploring conventional formulations such as drug-in-bottle, drug-in-capsule, or onsite compounding to ensure it reaches the clinic quickly
- Collaborative and joint analytical development for DS and DP to target a single method
- Adopting phase-appropriate development, manufacturing, and release strategy and ensuring the clinical supply material meets correct quality specifications
Leveraging the learnings to accelerate discovery to development
Bringing in the intended clinical critical product attributes in the early development phase ensures a fast transition from nonclinical animal studies to clinical trials in humans. This can be achieved through the following:
- Developability assessment from the lead optimization stage
- Salt/polymorph screening before tox
- DP specifications based on DS specifications (especially on impurities)
- Particle size distribution (PSD) and form controls for DS and DP
Another important step is choosing the dosage form and establishing the process that can be scaled up to the correct quality specifications quickly and cost-effectively. This can be achieved through the following:
- Solubility/permeability rate limited absorption assessment
- Defining a formulation strategy considering tox and FIH
- A common analytical method development for both DS and DP
- Risk assessment of nitrosamine and residual solvents at the DS stage
Right governance, along with project management
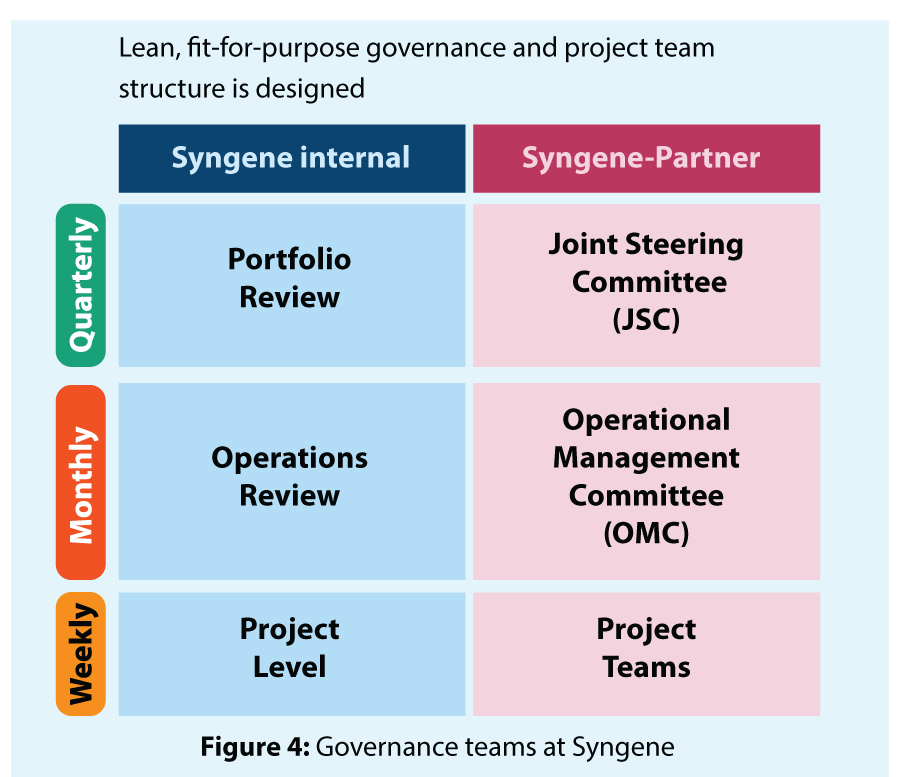
Right governance and project management can help accelerate programs by identifying opportunities to shorten time on critical paths and mitigating risks. Syngene has dedicated governance teams for CMC projects fortified by strong project management (Figure 4).
The governance team consists of Subject matter experts (SMEs) from each discipline with vast experience in handling integrated CMC projects. They evaluate the risk at each stage gate and drive the program in the right direction. The project management team manages project planning and risk assessment, realigning change requests, introducing operational efficiencies, and managing subcontracted activities (Figure 5).
The CMC lead drives the project, technically connecting all the internal stakeholders. He/she would be the point of contact for the client for all technical discussions and decisions.
Supply chain practices to accelerate FIH clinical supply and mitigate supply disruptions
To build robust and scalable supply chains of the future, CDMOs must prioritize supply chain resilience, compliance, and risk management to mitigate the risks. We have identified four major sources of supply risks that could disrupt the on-time delivery of shipments.
- Environmental
- Economic
- Geopolitical
- Supplier-based To counter these risks and stay ahead of the competition, we have put in place a strong business continuity plan that is integral to our supply chain (Figure 6).
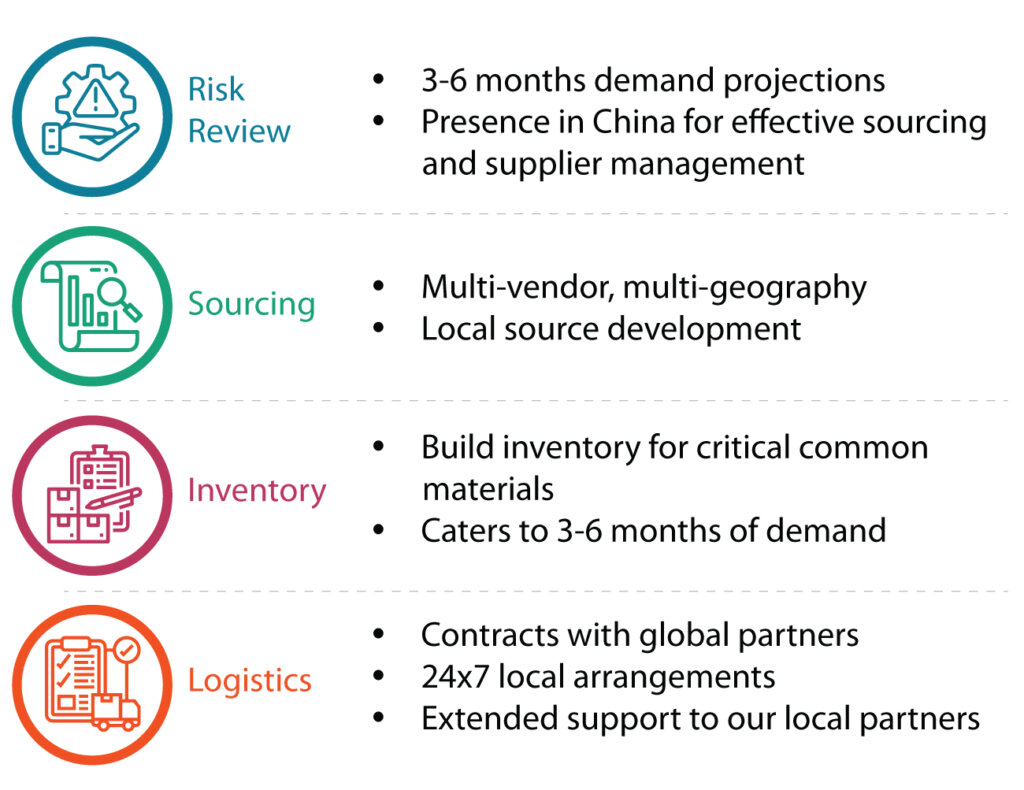
Supply chain practices to accelerate FIH clinical supply and mitigate supply disruptions
A risk mitigation strategy can help build better planning and predictability. It allows us to do the following:
- Build better planning and predictability
- Look for opportunities for backward integration
- Design process to avoid expensive/longer lead time material
- Develop supplier ecosystems and integrate information technology systems between the vendors
- Build a buyer ecosystem for general raw materials The risk mitigation strategy employed at Syngene at various stages of development is summarized in Table 1.
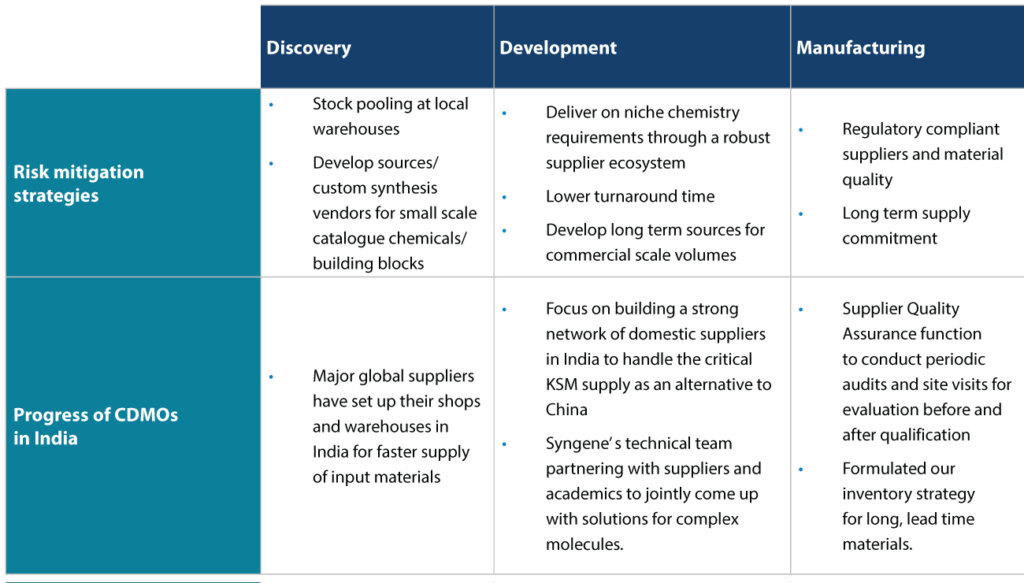
CDMO: Contract development and manufacturing organization; KSM: Key starting material
Syngene’s key differentiators
With a strong track record in CMC client projects around the globe, Syngene offers a one-stop solution for end-to-end Lead to FIH services. Our key differentiators are as follows:
- Integrated platform: An integrated platform for DS and DP development that leads to a significant reduction in timelines
- Knowledge sharing: Easy knowledge sharing of physical and chemical properties enables quick turnaround of formulation development
- Co-location: Availability of the same campus for varied activities makes the physical handover of materials possible without the need for import-export duty and license
- Project management: Clear development and manufacturing plan before project initiation. Complete plan designed only after a full risk assessment
- Risk assessment: Clear risk-assessed development and manufacturing plan before project initiation
- Regulatory experience: In-depth and wide regulatory expertise to expedite the development process
Conclusion
Drug development is a massive undertaking involving high risks at every stage. By partnering with leading CDMO Syngene, pharma companies can reap the benefits of a powerful business model that has end-to-end and integrated services at its core. With this, pharma companies can achieve effective integration across drug discovery, development, and manufacturing and implement phase-appropriate strategies for an increased chance of clinical success. Additionally, they get the advantage of expert regulatory support, robust governance, and a supply chain to ensure timely project completion. Together, it would help pharma companies mitigate risks and accelerate the lead to FIH journey of their small molecules, including seamless progression to commercial launch.
About the authors









