Introduction
Biophysics-driven understanding of higher order structures (HOS) and corresponding critical quality attributes (CQA) are imperative for drug regulation.
HOS modulates the functional repertoire of biologics which mainly comprises proteins. On the other hand, critical quality attributes (CQAs) are the physical, chemical, biological, or microbiological properties or characteristics that should be within an appropriate limit, range, or distribution to achieve the intended product quality (Source: ICH Q8 R2). CQAs typically relate to the drug component’s excipients, intermediates (materials in the process), and drug products.
In this Point of View, we discuss the role of biophysics driven analytical tools in accurately identifying biologics CQAs and analyzing HOS – resulting in the final product being efficacious and safe for patients from a regulatory perspective.
Challenges in developing biologics
Biologics are derived from live organisms. Hence their function, efficacy, and safety-related features depend on their production and processing conditions. Even minor adjustments to the manufacturing process can significantly affect the quality of a biological product. Other challenges in developing biologics include the following:
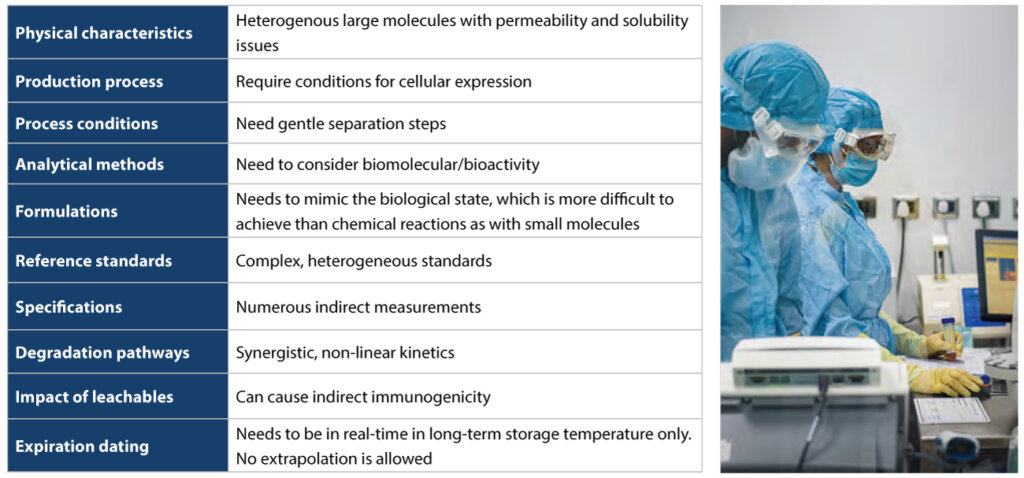
Regulatory role of CQAs in biologics
Based on ICH recommendations, regulators require information on a drug’s quality, safety, and efficacy. Since CQAs define the process which determines the drug product, the most important parameter in regulating chemistry manufacturing control (CMC) is the CQA. This is followed by preclinical and clinical testing to further establish the CQAs of novel molecules before NDA.

Challenges in developing biologics
CMC is crucial in the early phases of drug development. It includes all activities undertaken to ensure a pharmaceutical product’s safety, efficacy, and homogeneity. In the case of biosimilars, the data for the new drug is acquired based on the reference product, with CQA being a prerequisite for process control.
The CMC process is analyzed through the characterization of the pharmaceutical product under the following heads:
- Physicochemical properties
- Biological activity
- Specification
- Stability
Challenges in CQA analysis
While CQAs of small molecule pharmaceuticals can affect a drug’s purity, potency, release, and stability, the complexity of biologics can lead to the development of new quality attributes that can impact the safety and efficacy of the biologic. CQA analysis in biologics poses the following challenges:
- Clinical implications: Requires process understanding/ control/consistency to ensure the safety and efficacy of the drug
- Physicochemical understanding of CQAs: Requires understanding of aggregates and impurity profile of the drug
- Functional implication of CQAs: Requires understanding of target efficacy and safety of the drug
Syngene’s approach to Biologics CMC
Given the intricate nature of CMC, the most effective method for developing a concise strategy is to base it on a comprehensive target product profile. Syngene’s integrated CMC approach is as follows:
- Create a holistic target product profile by understanding CMC from the preclinical, clinical, and regulatory perspectives
- Put together a strategy to proactively identify possible issues and go about acquiring appropriate data sets to address those issues
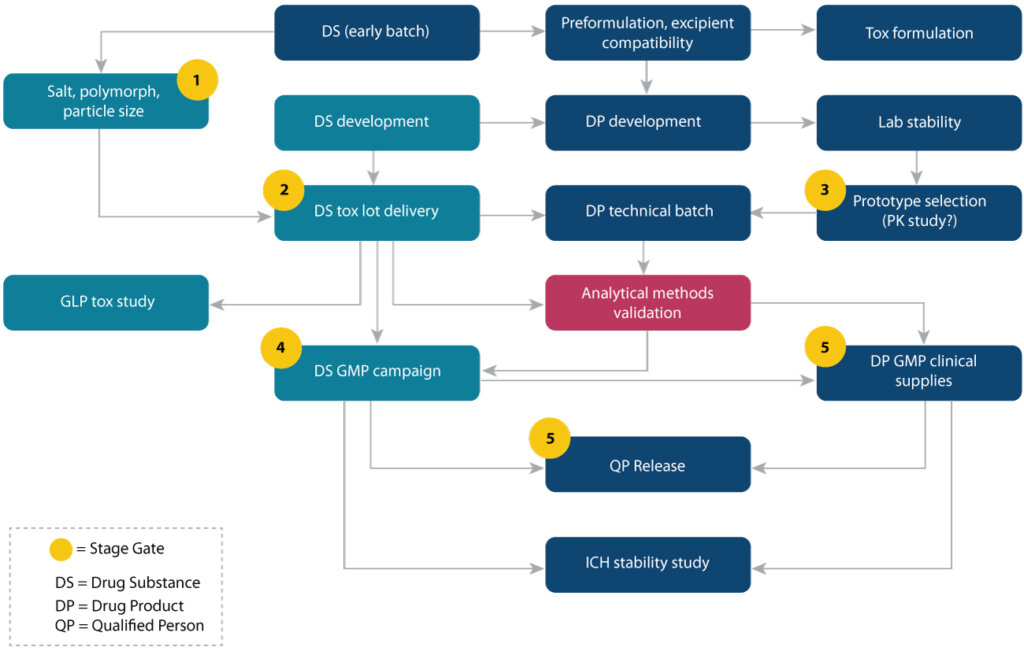
During this process, our team of world-class scientists uses a range of tools to analyze CQAs of biologics to ensure the final drug product is efficacious and safe from a clinical, physicochemical, and functional perspective
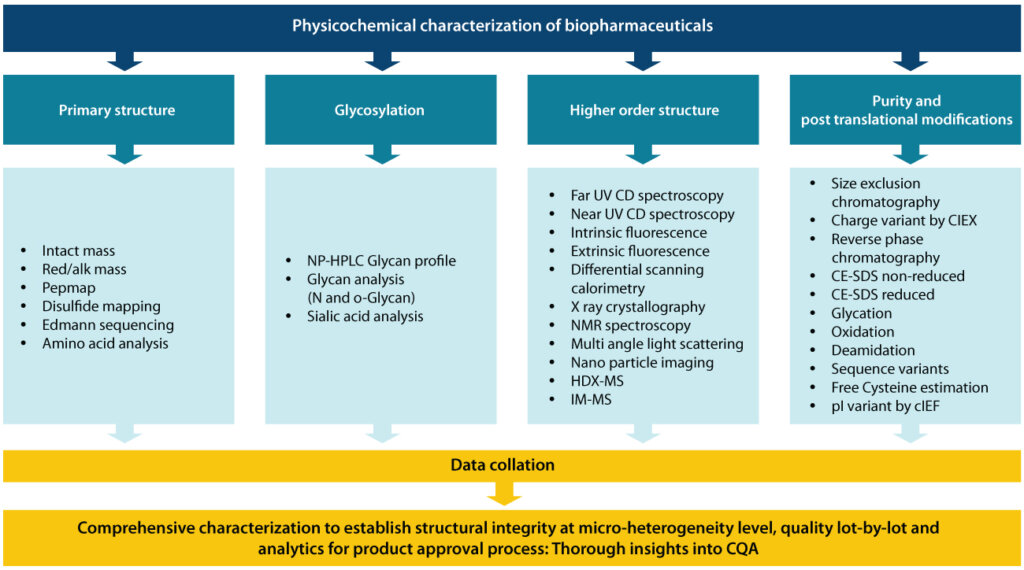
Understanding the structural characterization workflow of biopharmaceuticals
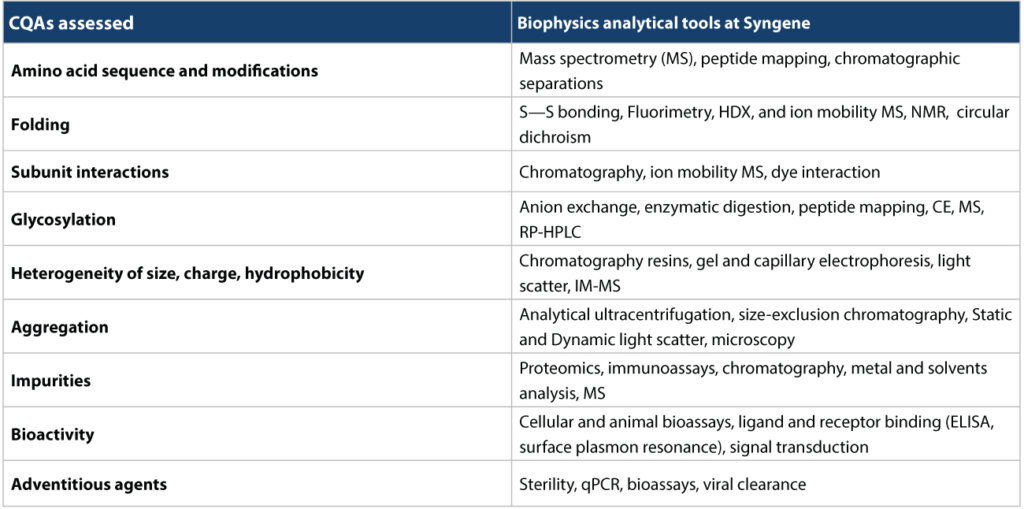
Structural insights using Syngene’s biophysics tools
Here are some examples of structural insights on various proteins derived from Syngene’s biophysics tools.
1. Intact mass analysis-intact/deglycosylated: Insights into the primary structure
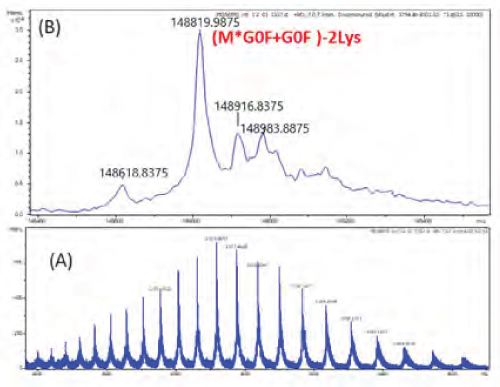
Mass spectrometry (MS) is a key technique that plays a primary role in the structural assessment of a molecule. Throughout all stages of mAb development and production, mass spectrometry-based methodologies are used to provide essential information on the primary structures, including amino-acid sequence, glycosylation, and other post-translational modifications. The intact mass analysis offers an unequivocal assessment of the molecular mass of an intact IgG1 mAb and other proteins, along with the intact level heterogeneity without prior enzymatic digestion. The heterogeneity includes post-translational modifications, drug antibody conjugates, and other degradation products
2. Peptide mass fingerprinting analysis: Insights into the primary structure
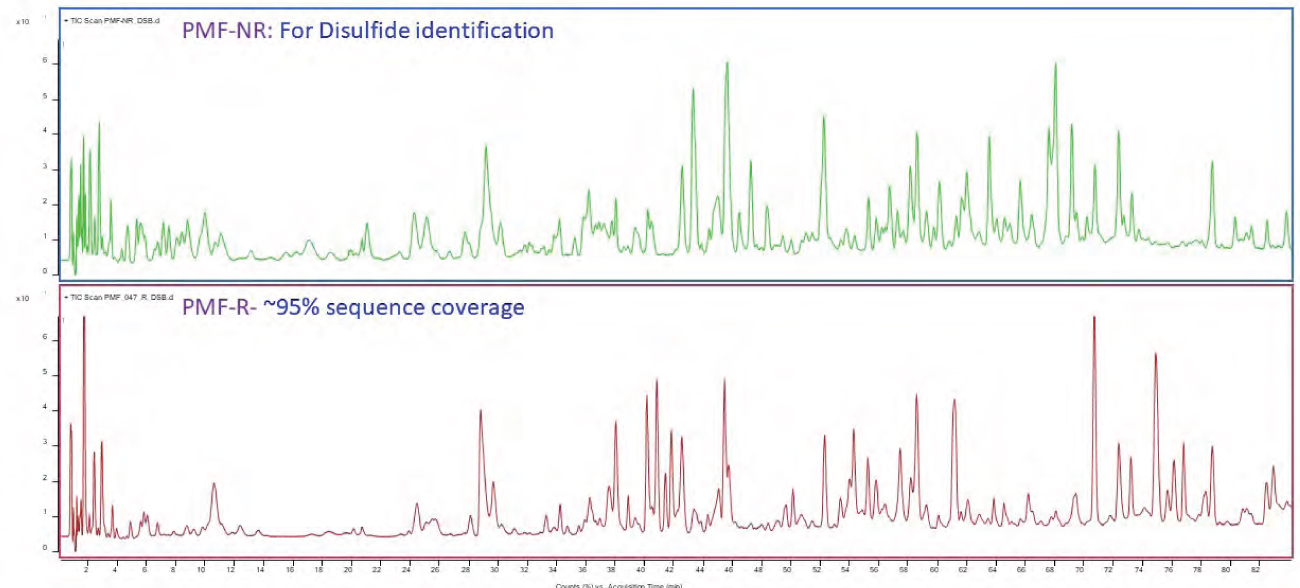
Peptide mass fingerprinting is a critical method in the characterization of biotherapeutic proteins as it elucidates the primary amino acid structure of the proteins. For recombinant protein pharmaceuticals, such as monoclonal antibodies (mAbs), peptide mapping is used as “proof of identity” of the molecule. Characterization of biologic drugs by peptide mapping elucidates not only its constituent peptide fragments but also ensures full sequence coverage of the biopharmaceutical molecule. Information on biotherapeutic drugs’ primary structure and impurity characteristics can be obtained using state-of-the-art, high-resolution mass spectrometry technology.
3. Glycan profile
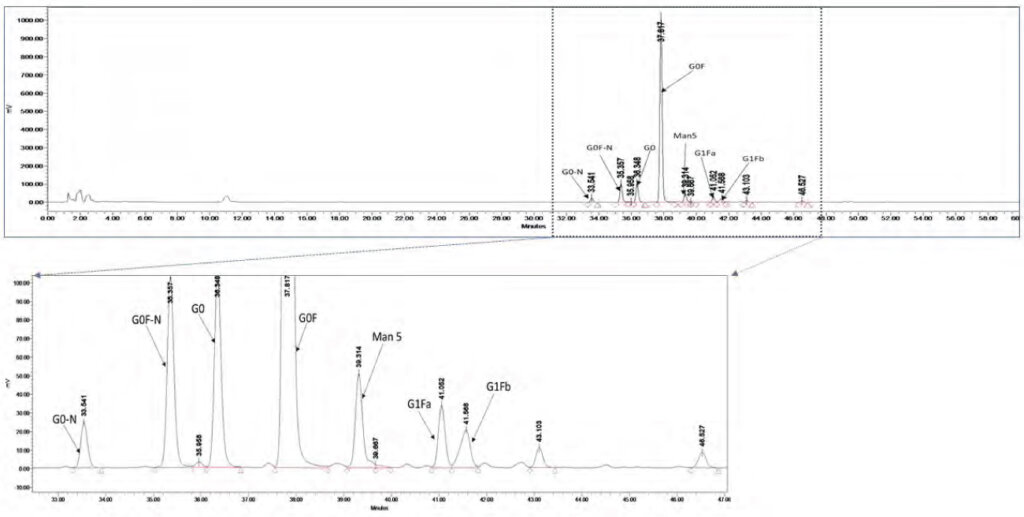
Glyco-molecules play a critical role in many physiological and pathological reactions ranging from immunity to blood clotting, cell death, and cell development. The majority of proteins targeted by therapeutic compounds are glycoproteins. Correct glycosylation is essential if the protein is to achieve and maintain the correct structure and therapeutic efficacy. Some biologics, including antibodies, must have glycans to create the desired medical impact. Hence it is imperative to have quantitative insight into the Glycan profile of such biologics.
4. LMMS confirmation in formulated drug substance (FDS) by SEC-MALS
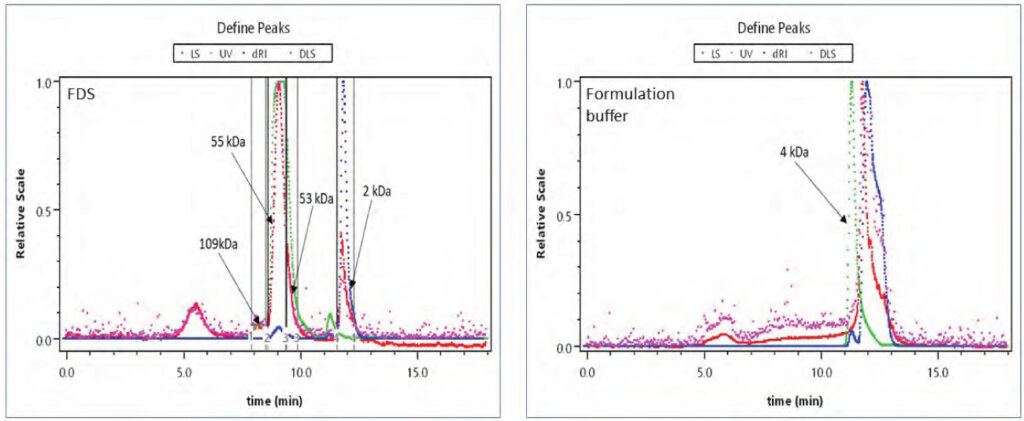
Aggregates are one of the key CQAs that need to be monitored during the lifecycle of a biologic. Amongst the various methods available, size-exclusion chromatography coupled with multi-angle light scattering (SEC-MALS) is a state-of-the-art method to get deep insights into the aggregation behavior of a protein biologic. As shown, the peak observed post the main peak (55 kDA) was confirmed as a low molecular mass species (LMMS). This was despite co-eluting with the excipient peak due to its difference in the size (2kDa peak) with respect to the excipient (4 kDa peak) using the MALS data for the corresponding SEC peak.
Case study
How Syngene analyzed the fluorescence intensity of a product and concluded on the filtration issue with the help of HOS analysis
Steady-state extrinsic or intrinsic fluorescence is a well-known tool for accessing the tertiary structure. Extrinsic fluorescence, where a dye-like ANS is used, gives insights into the hydrophobic regions of a protein. Hence, it is utilized to understand the presence or absence of aggregated species quickly. In this case study, the ANS binding-based extrinsic fluorescence analysis helped in the root cause analysis of challenges in product filtration.
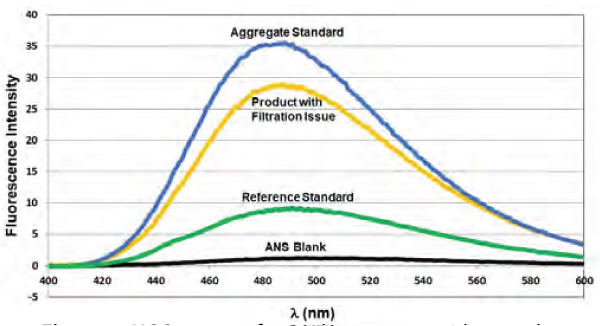
As shown in Figure 7, a substantial difference can be seen in the ANS binding analysis for the product that had a filtration issue. This indicates that more hydrophobic areas are exposed in the samples than in the reference standard (as shown by an increase in intensity). This suggests that aggregation, driven by hydrophobics, caused filtration difficulties.
Conclusion
Regulatory authorities expect thorough investigative structural analysis (including HOS) and in vitro functional analysis of a drug product before permitting human clinical trials. Biophysics and biochemistry help decipher the CQAs to ensure appropriate process control and consistency in manufacturing the final drug product. Syngene offers the biophysical analysis of pharmaceuticals and medications from early discovery (molecular biology) to cell line development and stability, process development, and process scale-up until commercial manufacturing. Our wide range of biophysics-driven analytical tools accurately characterizes the higher-order structure and corresponding CQAs to determine product quality and, consequently, the safety and efficacy of the drug product. This results in faster regulatory approval and reduced time-to-market for our clients. Further, Syngene maintains a consistent quality environment through stringent checks and balances across all phases of the drug discoverydevelopment continuum. Testament to this is the recent US FDA approval we received for our Biologics facility with zero 483 observations during the pre-approval inspection. In partnering with us, biotechs can rest assured that all deliverables meet the highest quality standards at all times.
About the author








