Introduction
Well designed and meticulously implemented preclinical toxicology studies are crucial for the success of any drug development program. Pre-clinical toxicology studies should reliably assess the safety of new drug entities, thus building a multi-faceted understanding of compounds to lay the groundwork for clinical trials and regulatory approval. The objectives of preclinical toxicology studies are as follows:
- Identify toxicity/delayed effects/reversibility in the target organ(s)
- Identify appropriate parameters to monitor in the clinic
- Identify potentially “at-risk” populations
- Determine NOAEL (no observed adverse effect level) and MTD (maximum tolerated dose)
- Determine the initial safe starting dose for phase 1 clinical trials
At Syngene, we have developed a roadmap that seamlessly integrates the various pre-clinical studies, thus accelerating the initiation of clinical trials.
Pre-IND, IND and NDA tox roadmap close integration between outsourced teams and their internal research.
Pre-IND, IND and NDA tox roadmap
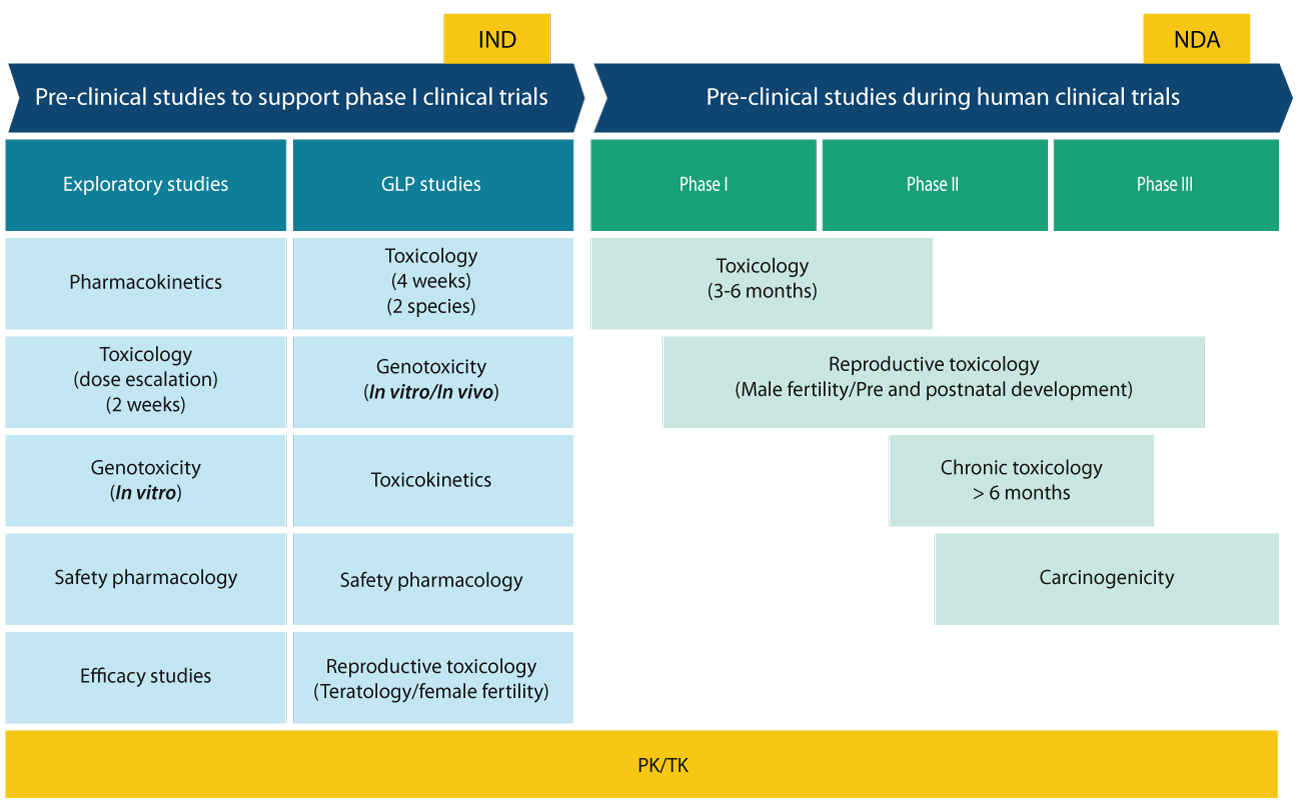
Pre-clinical studies start with exploratory pharmacology, pharmacokinetics and toxicology studies. These studies assess efficacy, systemic blood exposure, bioavailability and the toxicological liability of lead candidates. These studies include pharmacokinetics, MTD and dose range findings, mini Ames (to screen for the mutagenic potential of compounds) and the hERG assay. Safety pharmacology (hERG assay in particular) is critical at this stage as several drugs are withdrawn from market due to cardiotoxic effects. The next phase, GLP toxicology studies, is highly regulated and subject to a strict set of guidelines (OECD, FDA, ICH, EMEA, etc.). The material used in the definitive GLP studies needs to be GMP or GLP characterized by a Certificate of Analysis or equivalent. Pivotal 4-week GLP toxicology studies are carried out in one rodent (rat or mice) and one non-rodent (either mini pig, dog or monkey) species.
A battery of genetic toxicology studies is carried out either before this or concurrently, including AMES test, in-vivo micronucleus test, and chromosome aberration. Toxicokinetics is usually integrated into GLP toxicology studies. Validated analytical methods are required to determine the amount of drug in the serum/ plasma. Reproductive toxicology studies are conducted on a case-to-case basis, particularly for drugs or biologics that are meant for women of childbearing age (teratology study). Though pre-clinical studies may lead to the attrition of compounds during pre-clinical development or GLP toxicology phase, this helps save millions of dollars during the drug development program.
Key considerations for designing the optimal pre-clinical strategy
Dose formulation: Dose formulation is critical due to its effect on the release and absorption of the compound and due to its impact on the pharmacokinetic profile of the compound. The formulation developed during the early discovery phase is generally designed to maximize exposure of compound and selected based on factors such as solubility, stability, the dose volume to be used, and tolerability of the vehicle in the nonclinical species to be used. However, in the GLP toxicology phase, three different types of formulations may be required; one to support in-vivo toxicology studies; the second to support genetic toxicology studies; and the third, hERG buffer formulation using a physiological salt solution with the minimal solvent. Fully validated methods for formulation analyses is a critical requirement for GLP toxicology studies.
These validated methods are used primarily for dose confirmation analysis and for demonstrating the accuracy of dose concentration in dosing formulations. Species selection: Species selection is made based on in-vitro metabolic profile/ ADME properties and bioavailability for small molecules. For large molecules, relevant species are considered i.e. species in which the molecule is pharmacologically active. Historically, two species have been used for pre-clinical testing. Rat and dog are usually used due to the availability of abundant historical data. Other species may be used if data suggests that they are more appropriate and biologically relevant. Non-human primates and minipigs are recognized, alternative models. There’s increasing use of minipig models over dog models. Syngene recently completed an IND program for a client in which minipig was used as the non-rodent species. A complete pre-clinical package including CMC and medical write-ups was prepared and submitted based on the minipig model, ultimately leading to approval and initiation of clinical trials.
IND GLP tox & safety pharmacology – indicative timelines
Through the rational design of a comprehensive pre-clinical tox roadmap, we have been able to significantly reduce the time needed to complete the required studies:
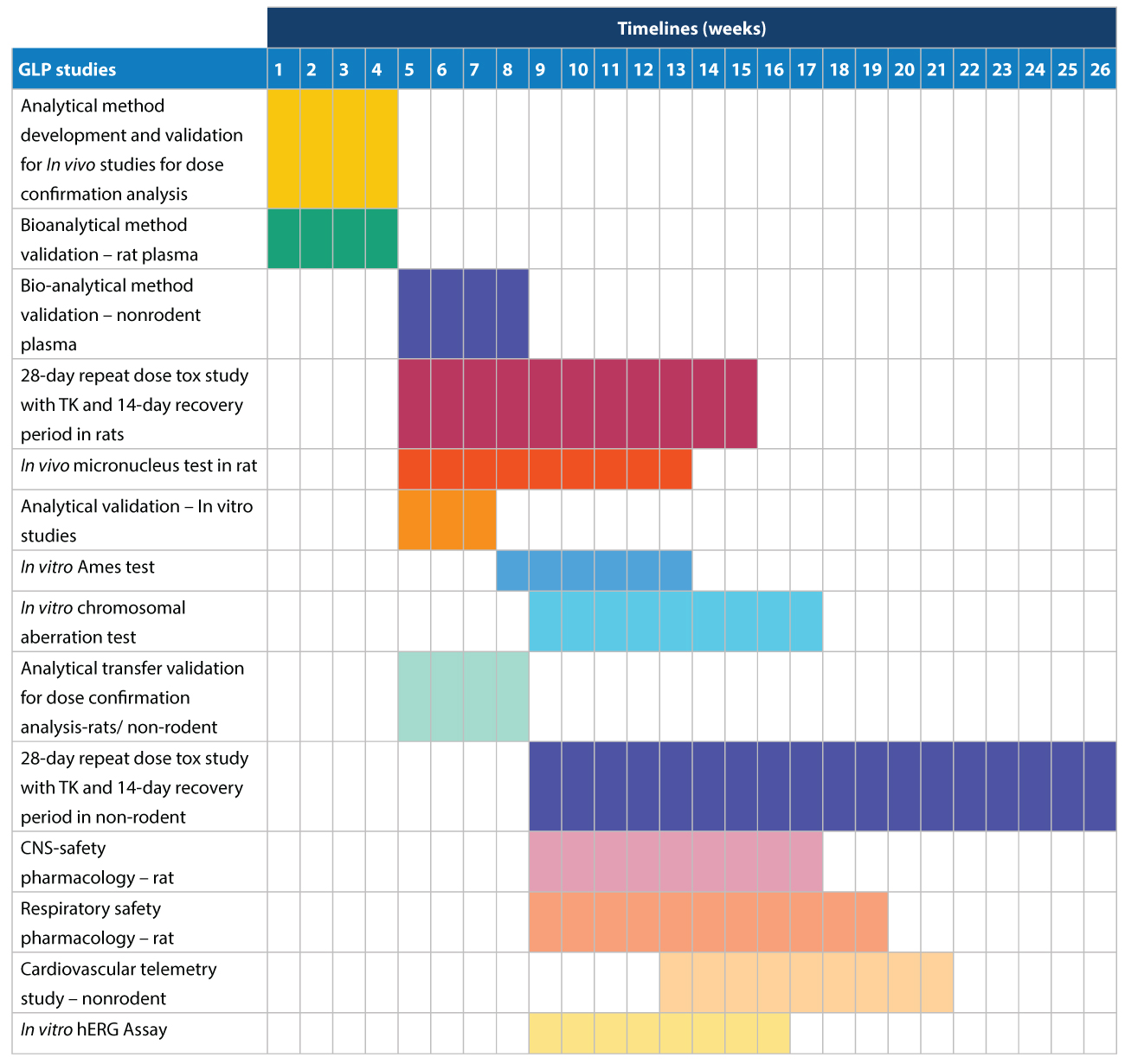
Our approach also ensures regulatory compliance and optimizes the chances of success for the drug development program, while reducing
the risk of attrition further along the lifecycle of the clinical candidate(s).
Syngene – Your strategic partner for pre-clinical studies
Even though preclinical studies are complicated and time consuming, they act as a bridge between the discovery of a new drug compound and FIH testing. Pre-clinical studies help in selecting the right compound, the right formulation and the optimal drug delivery system to reduce the risk of harmful effects on humans. Syngene brings to the table 25+ years of experience in pre-clinical studies, with a team of toxicologists, pathologists, medicinal chemists, regulatory specialists and support personnel who have in-depth understanding of preclinical toxicology. We are fully equipped to identify potential risk factors early in the drug development process and to ensure the success of your drug development programs.
About the author








