Introduction
Since the establishment of Chinese Hamster Ovary (CHO) cells in the 1950s, the production of therapeutic proteins has seen continuous innovation and growth. The potential of therapeutic proteins, such as antibodies, bispecifics, antibody-drug conjugates (ADCs), and recombinant proteins, is driving the demand for high-titer clones. Biopharma companies require optimal clone yields to ensure maximum concentration and effectiveness of these therapeutic proteins. High titer capability is essential for delivering superior results and meeting the escalating needs of biopharma production. Additionally, the rapid pace of innovation in this sector necessitates shortened development and production timelines. A streamlined platform enables companies to accelerate their processes, ensuring quicker market entry for new therapies, which is vital in a fast-evolving landscape. Enhancing manufacturability is crucial for reducing the risks associated with scaling production. A platform that produces clones with high manufacturability allows for effective and efficient scaling, minimizing potential hurdles in the manufacturing process.
The SynWeave™ Platform: Leveraging Transposon Technology and Expert Process Development
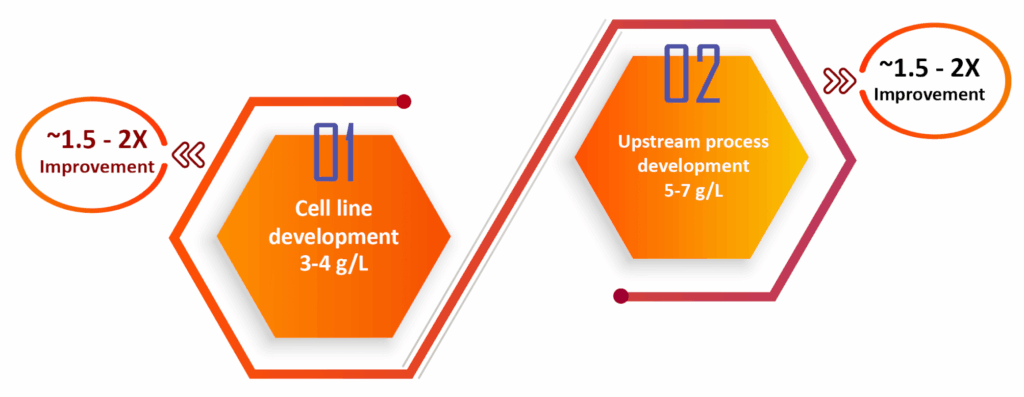
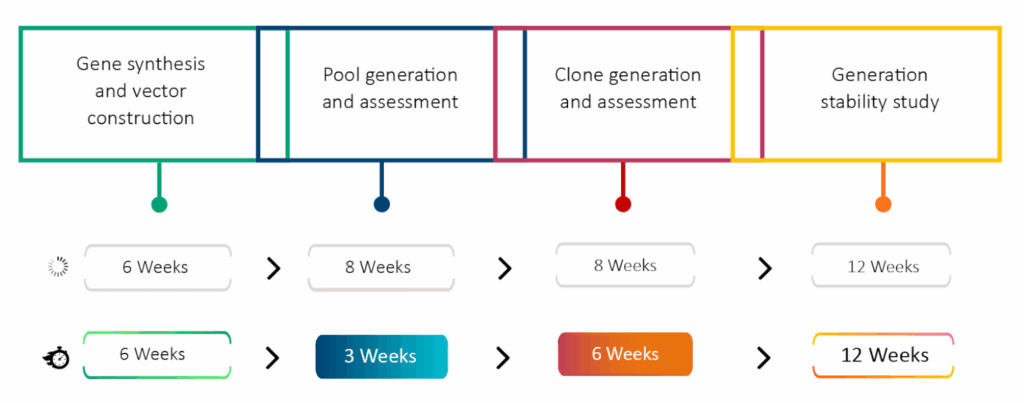
The SynWeave™ platform at Syngene offers various methods to enhance the titer of therapeutic proteins. Our process begins with cell line development using transposon-based technology, achieving titers of 3 to 4 grams per liter with generated monoclonal antibodies. This technology, licensed from a European-based contract research organization (CRO), is complemented by our upstream process development platform, resulting in an additional 1.5 to 2-fold increase in titer. Our titer enhancement platform includes transposon-based cell line generation using proprietary cell lines, significantly reducing the timeline for developing high-yielding, homogeneous clones. The platform minimizes experimentation through Design of Experiments (DoE) and the AMBER 250 bioreactor system, ensuring consistent product quality through a first time-right and expedited process.
Cell Line Development Workflow
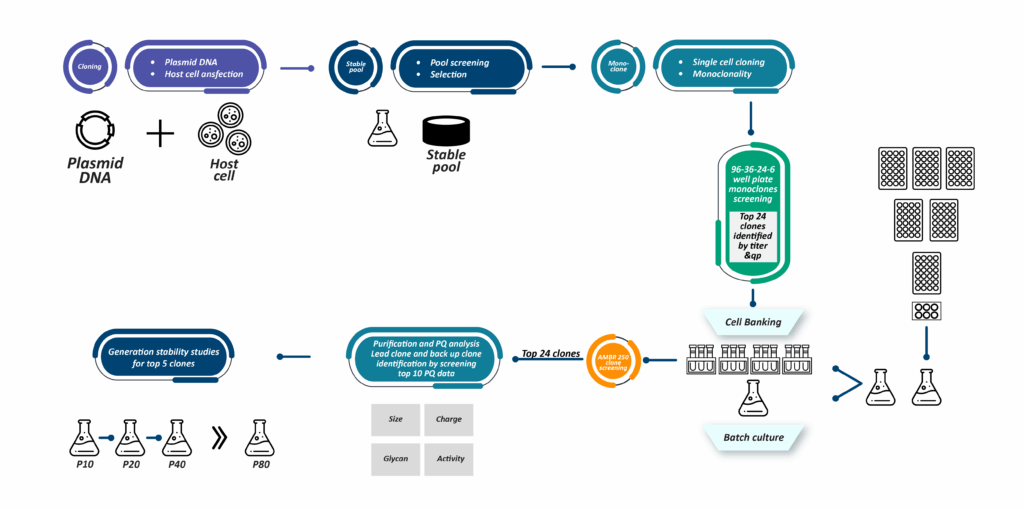
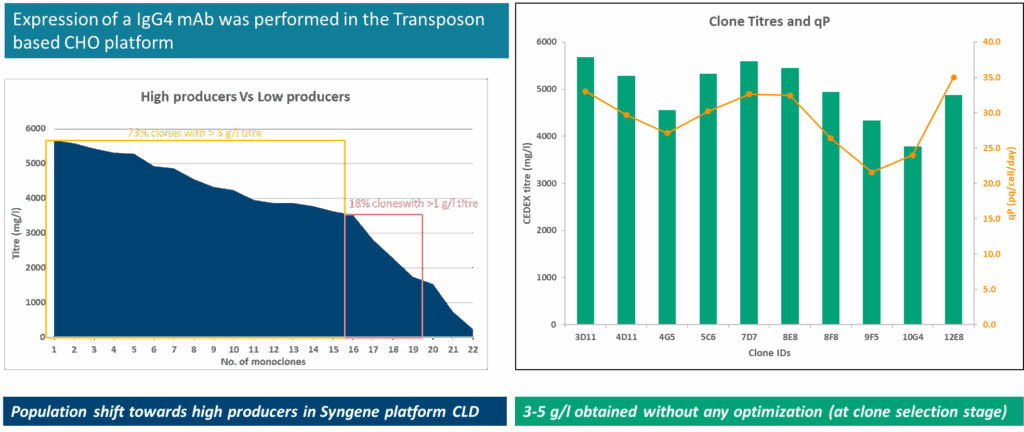
Our cell line development (CLD) workflow begins with codon-optimized genes for high expression. After pool selection, we perform single-cell cloning using the Cytena system, which dispenses individual cells into 96-well plates. Images captured during this process provide proof of monoclonality, confirming that the cell line originated from a single clone. For Fc-containing proteins, such as immunoglobulins or bispecific antibodies, we utilize the OCTET-HTX system for titer estimation. The AMBER-based mini bioreactor system ranks clones based on titer and quality parameters, including charge, monomer composition, glycan profile, and potency. Selected clones are preserved in liquid nitrogen canisters for future use. Our observations indicate that 70% of the clones we screen exhibit high titers, saving significant time and reducing the cost of raw materials. Using the SynWeave™ platform, we have obtained titers ranging from 3 to 5 grams per liter for IgG1 molecules without any optimization.
Stability and Consistency
In the biopharmaceutical context, stability is defined as the reproducible yield and quality of protein from a given cell line over an extended period. We generally assess the stability of research cell banks for up to 60 generations. Our observations indicate that clones generated using transposon technology maintain consistent product quality across generations, with no significant drop in titer. Typically, clones are considered stable if there is less than 30% variability in titer across generations. However, in our case, the decrease in titer across sixty generations is minimal, and the product quality remains highly conserved.
Upstream Development Workflow
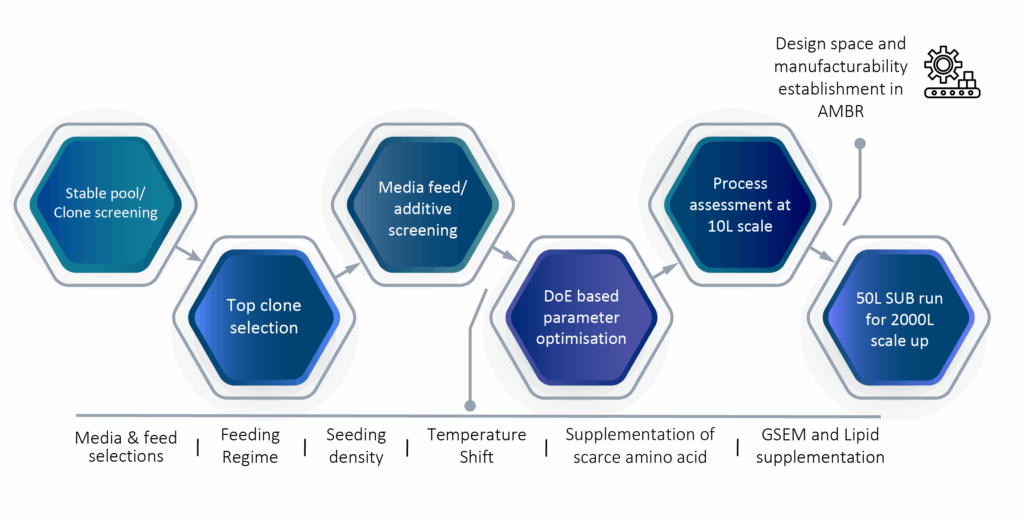
Our upstream process development utilizes the AMBER 250 system, a miniature bioreactor designed for screening optimal producers in a real manufacturing control environment. We monitor various offline parameters and use a Design of Experiments (DoE) approach for process development. This involves media feed screening, agitation, temperature downshifts, feeding regimes, and initial seeding density. Once the design space is confirmed, we scale up to a 10-liter system to validate the process. Media feed selection significantly impacts product expression and quality. We perform clone screening using the most suitable media and feeds for the target molecule, integrating media and feed screening into our top clone selection. Parametric studies, along with optimized mediafeeds and supplements, help establish the optimal process conditions.
Titer and manufacturability
Using the SynWeave™ platform, we have successfully shortened timelines, enhanced manufacturability, and increased titer in our biopharmaceutical production processes. This streamlined platform enables companies to accelerate their workflows, ensuring quicker market entry for new therapies—an essential factor in today’s rapidly evolving landscape. By improving manufacturability, we reduce the risks associated with scaling production, allowing for effective and efficient scaling that minimizes potential hurdles in the manufacturing process. For instance, in a case study involving the upstream process optimization of an in-house developed clone, we employed a Design of Experiments (DoE) approach to screen 24 bioreactor conditions, identifying optimal parameters for clone growth and medium compositions. This top condition was then scaled up to a 10-liter bioreactor, resulting in a titer of 7.2 grams per liter—a four-fold increase compared to previous methods. Similarly, for a client-provided cell line with an initial process duration of 22 days and a titer of 0.8 grams per liter, we implemented media feed screening and parametric interactions to reduce the process time to 17 days while increasing the titer to 4.2 grams per liter. This process was subsequently scaled up to 500 liters, achieving a consistent titer of 5 grams per liter and significantly reducing the GMP manufacturing timeline. Read more in our case study (link to four-fold titre)
SynWeave™ in a Nutshell
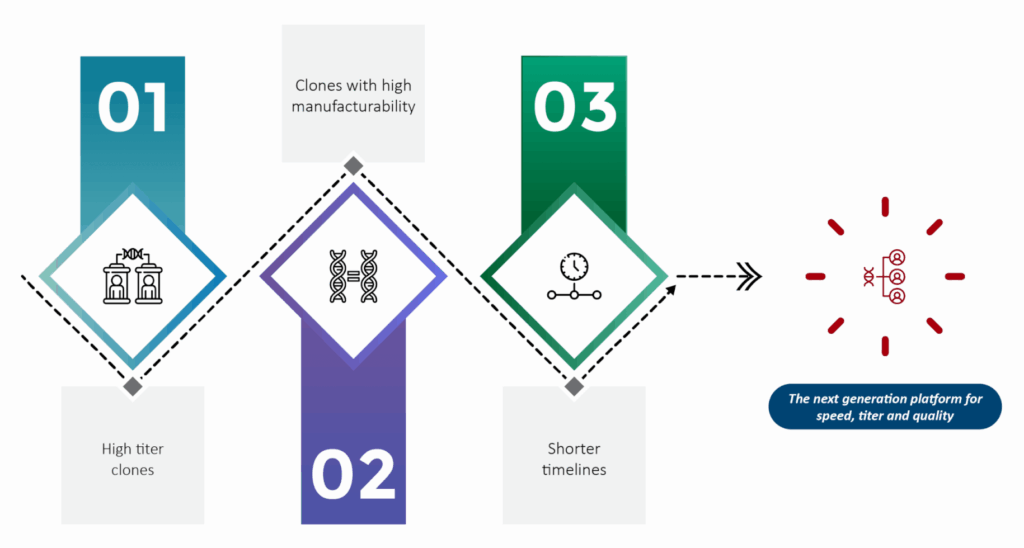
SynWeave™ was designed to significantly improve the biopharma manufacturing process through three core advancements:
- Producing High Titer Clones: Optimizing clone yields to achieve maximum concentration and effectiveness.
- Shortening Timelines: Streamlining development and production timelines to bring solutions to market more quickly.
- Creating Clones with High Manufacturability: Enhancing manufacturability to reduce the risks associated with scaling production.
The development of SynWeave™ combines transposon-based gene expression technology with the extensive process development experience of Syngene’s scientific team. We are also working on N-1 perfusion (link to our perfusion viewpoint) with high cell density fermentation batches to further increase titer, with data indicating a twofold increase in titer through N-1 perfusion.






