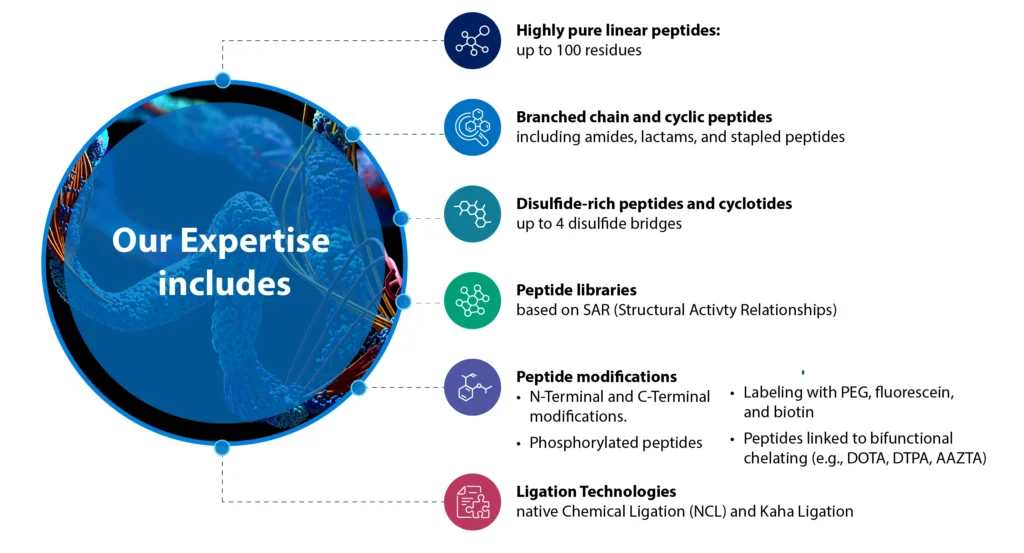
Syngene has considerable experience in peptide development including state-of-the-art analytical instruments for peptide purification and characterization. Our experience in Peptide development is as follows:
- Linear peptides: Up to 50 residues, up to g scale, purity > 95%
- Disulfide rich peptides (linear and cyclotides)
- Library of 50 compounds (20-50 mg, >95%)
- Branched chain peptides
- Cyclic peptides: Amides, lactams, disulfide bridge, stapled peptides
- N-Terminal and C-Terminal modification
- Phosphorylated peptides
- PEG, fluorescein, biotin labeled peptides
- Peptides linked to bifunctional chelating agents such as DOTA, DTPA and AAZTA
- Ligation: NCL and KAHA ligation
End-to-end Peptide services: Early Discovery to cGMP Manufacturing
Peptides are a class of therapeutic molecules with unique biological properties and a growing presence in modern medicine. However, the development of peptide therapeutics presents unique challenges, including synthesis, purification, stability, and delivery.
Syngene offers a comprehensive, integrated suite of services to navigate these complexities, from early discovery to clinical supply. Our goal is to accelerate your peptide drug development with efficiency, quality, and cost-effectiveness.
Syngene provides a wide range of custom peptide synthesis services to meet your specific research and development needs. Our expertise spans various types of peptides and modifications, ensuring high purity and quality at every scale.
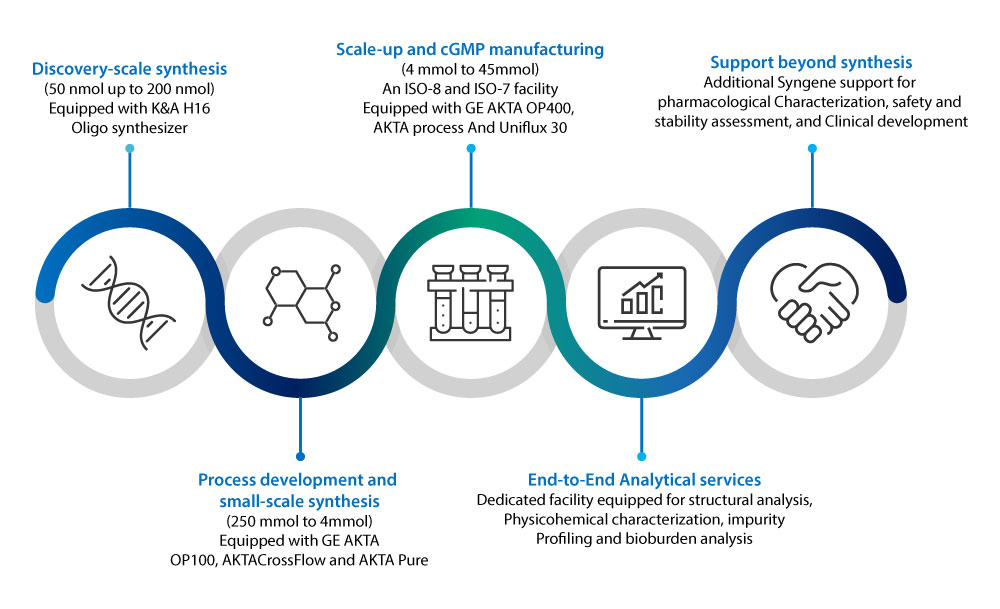
Integrated & Accelerated Program
Our integrated program is designed to streamline the entire peptide drug development lifecycle. By co-locating development workflows for both Drug Substance (DS) and Drug Product (DP) within a single facility and under one quality system, we facilitate seamless knowledge transfer and significantly reduce timelines. This parallel approach enhances efficiency and provides a faster path to clinical and commercial supply.
Syngene’s Center of Excellence for peptides
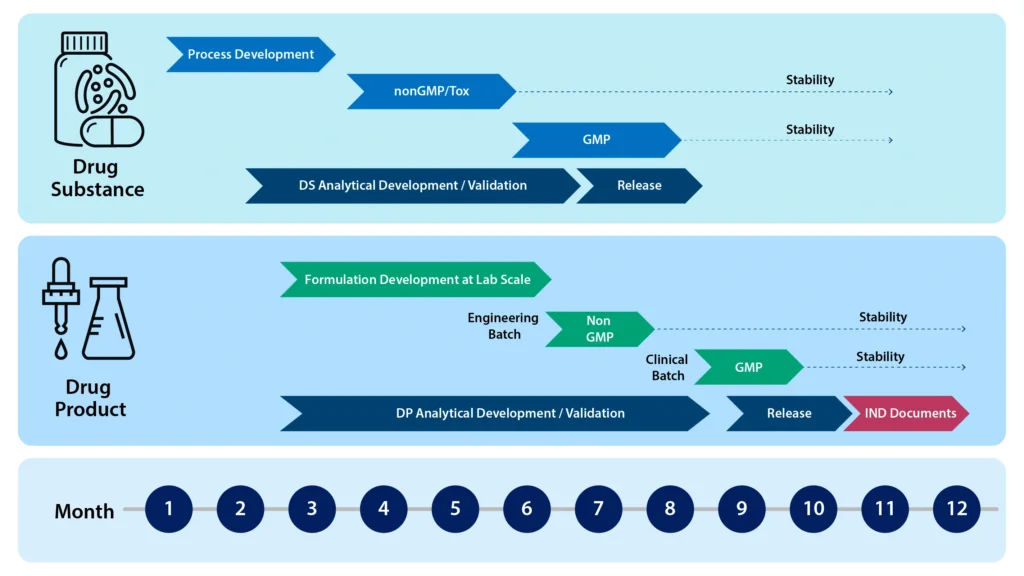
The Syngene peptide team has a wealth of experience synthesizing complex peptides. Our Center of Excellence for peptides offers end-to-end services ranging from Discovery to Development and cGMP Manufacturing. The figure below shows the time efficiencies that biopharma companies can gain by partnering with CROs/CDMOs like Syngene having an integrated workflow.
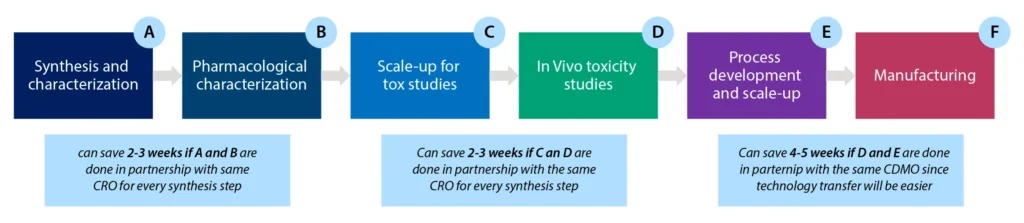
Accelerating Peptide supply by Integrating Drug Substance (DS) and Drug Product (DP)
Syngene has used its wealth of experience and knowledge in Peptide API development and manufacture (DS), as well as Clinical / Commercial drug product (DP) development and manufacturing, to create a fully integrated program for clinical / commercial supply. The figure 5 shows how we can perform development workflows in parallel in the same facility, and under the same quality system. The enhanced knowledge sharing and consolidation of activities speeds up the development cycle to provide clinic or commercial ready Peptide Drug Product.
Why Partner With Syngene?
- Proven Track Record: Our team has extensive experience in peptide chemistry, from discovery to manufacturing.
- Integrated Center of Excellence: We offer a single-source solution for end-to-end services, ensuring a smooth and efficient transition between development stages.
- Flexible Synthesis Scales: We can accommodate projects from discovery scale to full cGMP manufacturing.
- Dedicated Analytical Facility: Our state-of-the-art analytical lab provides comprehensive structural analysis, characterization, and impurity profiling to ensure product quality.
- Time & Cost Efficiency: Our integrated workflows enable rapid and cost-effective development, allowing you to bring your therapeutics to market faster
Accelerating Peptide supply by Integrating Drug Substance (DS) and Drug Product (DP)
Syngene has used its wealth of experience and knowledge in Peptide API development and manufacture (DS), as well as Clinical / Commercial drug product (DP) development and manufacturing, to create a fully integrated program for clinical / commercial supply. The figure below shows how we can perform development workflows in parallel in the same facility, and under the same quality system. The enhanced knowledge sharing and consolidation of activities speeds up the development cycle to provide clinic or commercial ready Peptide Drug Product. Primarily aimed at the exciting Radio-Theranostic peptide space, we can also cater for much larger integrated peptide and peptide conjugate projects as well.






