Overview
A global pharma company partnered with Syngene to manufacture ~250 kg of their oncology compound using their technology pack. Early into the manufacturing process, Syngene observed that the client’s reaction process consumed high volumes of starting material and solvents while returning low yield with high wastage. In eight weeks, Syngene modified the process to adopt a Green route and scaled it for GMP manufacturing as well. Consequently,the pharma company reduced manufacturing costs by 30%, improving yield and reducing wastage.
The requirement
A global pharma company was looking for a partner to develop a robust, scalable, and safe manufacturing process for its oncology drug compound. The compound had initially been designed by the client’s offshore Medicinal Chemistry team. The client wanted Syngene to manufacture ~250 kg of the compound for use in Clinical Studies.
The challenge
The project comprised four chemical reaction steps (Boc-deprotection, Buchwald- Hartwig Cross-Coupling reaction, acid amine coupling, and ester hydrolysis) involving significant amounts of solvents and reagents at every level.
The client shared the reaction process to be used for manufacturing the compound. When executing the process, our team observed that ~3 kg of starting material was required to produce just 1 kg of the product. The process consumed more than ten volumes of solvent and had a low conversion rate of below 50 Area%. The isolated product also yielded less than 40 weight% despite consuming high quantities of starting material. At this rate, the client would need higher volumes of starting materials and solvents and be prepared for low yield and increased product costs.
For Syngene, this was an apt case for adopting Green practices in the design and synthesis of the API. However, integrating sustainable practices without sacrificing product quality, regulatory compliance, or commercial viability is challenging. Overcoming these issues requires the combined effort of R&D, process engineering, analytical, and production teams, not to mention leadership commitment to promote sustainability in the drug development processes.
The Solution
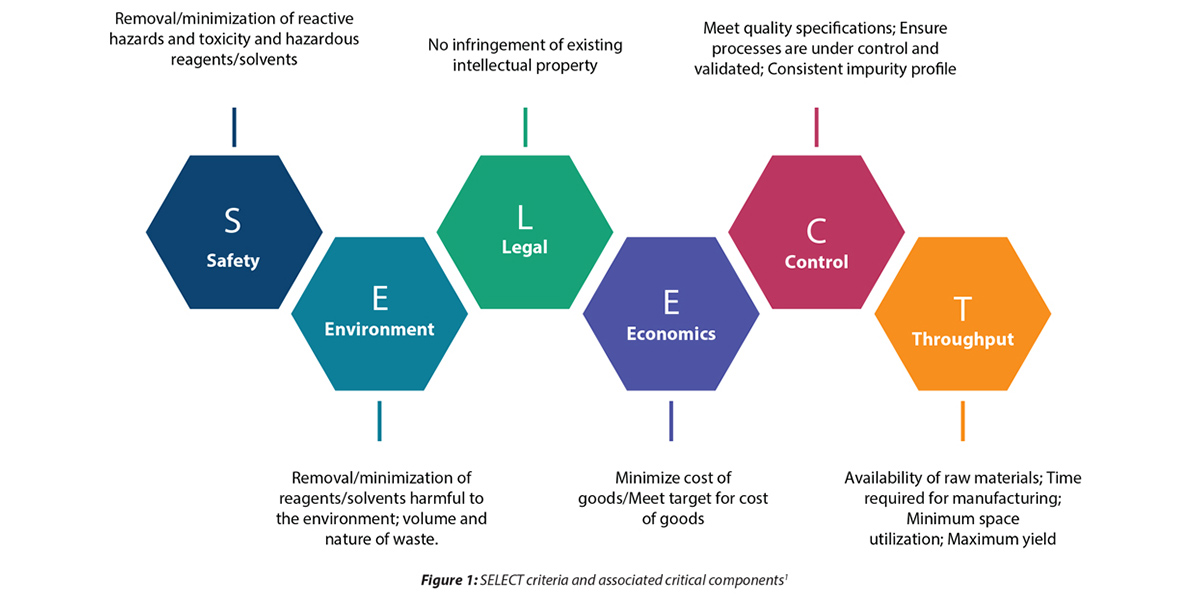
After discussing with the client and Syngene’s leadership team, we decided to take up the Green challenge in the interest of sustainability. A cross-functional team of highly skilled process chemists, analysts, process engineers, safety engineers, and quality, production, and project management teams came together to develop a holistic approach to drug development in keeping with Green principles. The current process was analyzed in detail. All the challenges regarding safety, environment, yield, impurity profiles, reproducibility, scalability, and process economics were listed based on the Safety, Environment, Legal, Economics, Control, and Throughput (SELECT)1 criteria. A detailed plan was developed with alternate routes based on the latest advances in published journals and brainstorming with the team.
The plan was divided into five sections– process development and optimization, analytical method development and validation, process safety assessment, and process engineering and scale-up. We decided to start with the most common and practical tool, the SELECT criteria, comprising selection guidelines, Green Chemistry metrics, life cycle assessment (LCA), the 3R principle of reduce, recycle, and reuse, etc. Additionally, we implemented our in-house tools, such as the Process Excellence tracker and Process Safety information, to help us better understand the client’s specific requirements.
Our process plan based on the SELECT criteria is depicted below.
The table below depicts the results achieved using Syngene’s modified Green process versus the client’s original process.
Step No | The client’s original process | Syngene’s modified Green process |
|---|---|---|
Step 1 | 1) Tertiary amine used as a base, and less than 40 Area% of product formation observed during the in-process control reaction 2) More than ten volumes of solvent used 3) ~ 3 kg of raw material used to deliver 1 kg of compound | 1) Amide used as a base and almost double the original process Area % product formation observed during the in-process control reaction Three volumes of solvent used 2) Double % yield obtained after isolation compared to the original process 3)~1 kg of raw material used to deliver 1 kg of compound |
Step 2 | ~ 5 volumes of process solvent used during the reaction process | ~4 volumes of methanol solvent used during the reaction process |
Step 3 | More than ten volumes of solvents used | ~7 volumes of solvent used~ 10 weight % increase compared to the client process. |
Syngene’s new modified process increased the yield by ~10 weight % compared to the client’s process. However, there was still scope for improvement. To achieve this, we further minimized the usage of key raw materials so as to convert the product in stage 1 itself. As a result, we were able to bring down overall product costs by 30 %.
Conclusion
Syngene’s new process for going Green proved a success. We not only reduced consumption of starting material and solvents but also reduced wastage, improved yield, and lowered overall product costs.
Our team developed the entire process in just eight weeks in keeping with project timelines. Initially, we delivered 100 grams of the API as proof of concept, along with a technology pack. Later, we scaled the process to achieve ~250 kg of API during cGMP manufacturing.
Syngene’s expertise in Green Chemistry (particularly solvent and cost-reduction, atom economy, waste reduction, and process efficiency) can help clients transform their businesses into more sustainable ones. By partnering with us, pharma companies can make use of modified industrial processes that work in harmony with the environment while maintaining economic viability at the same time.
To learn more about our Green Chemistry initiatives, contact our experts.
References
1Butters, M., Catterick, D., Craig, A., Curzons, A., Dale, D., Gillmore, A., & White, W. (2006). Critical assessment of pharmaceutical processes: a rationale for changing the
synthetic route. Chemical Reviews, 106(7), 3002-3027