Challenges in setting permitted daily exposure limits for pharmaceuticals: A review
Publication: National Library of Medicine; International Journal of Risk & Safety in Medicine
Approaches for setting occupational exposure limits in the pharmaceutical industry
Publication: Journal of Applied Toxicology Wiley
Predicting genotoxicity and carcinogenicity of drugs and chemicals using using OECD QSAR, DEREK Nexus® and TEST
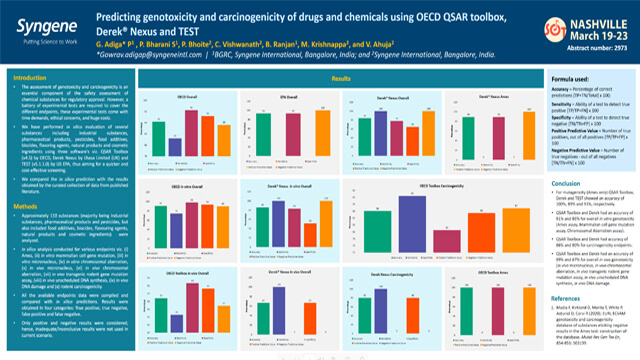
A comparison of in silico prediction of genotoxicity and carcinogenicity of drugs, with the results obtained from curated data in published literature.
How Syngene’s tech transfer approach helped a biotech company fast-track NDA approval for a novel compound to treat infections

Learn how Syngene ensured 100% first-time-right technology transfer for manufacturing limited number of batches of a novel compound to treat infections, including getting NDA approval in less than two years.
Regulatory role of Biophysics in Biologics CMC

Learn the role of biophysics-driven analytical tools in accurately identifying biologics CQAs and analyzing HOS to make the final product efficacious and safe for patients from a regulatory perspective.
How Syngene screened inhibitors of protein-protein interaction in HTS mode for accelerated drug discovery

Learn how Syngene screened 50,000 compounds using high throughput screening against two different transcription factors for accelerated drug discovery.
How Syngene accelerated drug discovery against a protein target using HTS
Learn how Syngene screened a library of 200,000 compounds using high throughput screening for a single protein target for accelerated drug discovery.
Robust and high-yielding platform process for plasmid DNA production

How Syngene scientists developed a platform process for plasmid DNA production with high titers of > 1 g/L upstream with >25% recovery downstream.
Process development and scale-up of highly challenging biomolecules

Three case studies on downstream platform process development for antibody fragments, three broadly neutralizing antibodies (bNAbs) against HIV, and a new class of regenerative implant used for treating a degenerative disease.
Mycoplasma Contamination Detection in Biopharmaceuticals
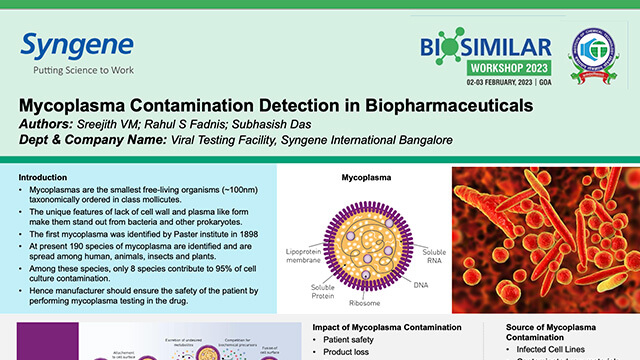
Presenting impact of Mycoplasma contamination, available testing methods, and why Syngene prefers NAT-based Mycoplasma detection method over other methods.






