Comprehensive solutions for Clinical Development of generic drugs

Synene has developed more than 100 assays for conducting BA/BE studies across small molecules and biologics.
Commercial Manufacturing services

Syngene supports manufacturing of regulatory starting materials, APIs, HPAPI, NCEs and novel advanced intermediates
Complimentary techniques for determining relative response factor of non- isolated impurities

Understanding the concept behind determining UV RRF of non-isolated impurities using High Performance Liquid Chromatography and Nuclear Magnetic Resonance
The many benefits of outsourcing Stability: A Baxter-Syngene Case study

Learn why Baxter chose to partner with Syngene for Stability studies, the business flow, governance mechanism and metrics that were implemented, which enabled a mutually successful collaboration.
Audit Syngene Virtually

Syngene is using virtual audits to enable clients/regulators experience its capability and infrastructure, without physically visiting the site.
Multidrug combo for companion animals

Learn how Syngene developed and commercialized for its client, a complex palatable dosage form comprising three actives for use in companion animals.
Combating COVID-19 – The Oligonucleotide Way

Synthetic oligonucleotides have emerged as an important modality for diagnostics and potential therapy option for COVID-19.
Development of degraders: A Medicinal Chemistry perspective
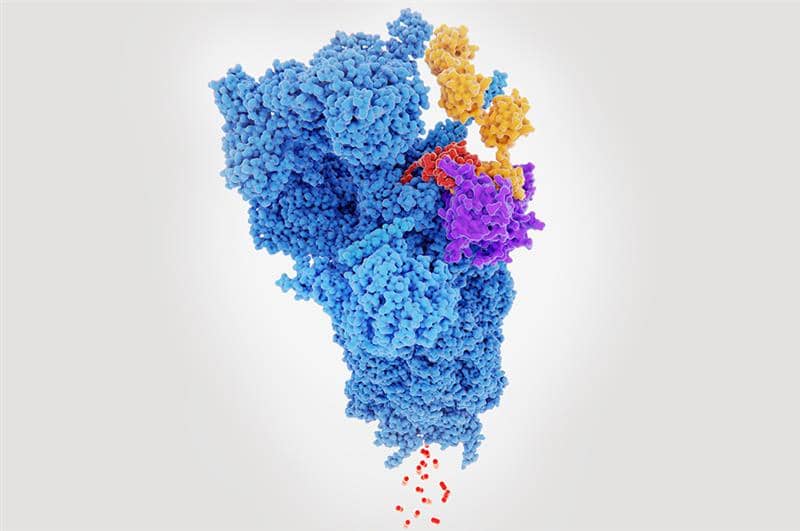
Key considerations for developing protein degraders from a medicinal chemistry perspective regardless of the mechanism of modulation.
Has the Age of Antisense Oligonucleotides Finally Arrived

Antisense Oligonucleotides is gaining momentum as a treatment modality to correct faulty protein expression by non-conventional means i.e., splicing modifications.
Harnessing Targeted Protein Degradation
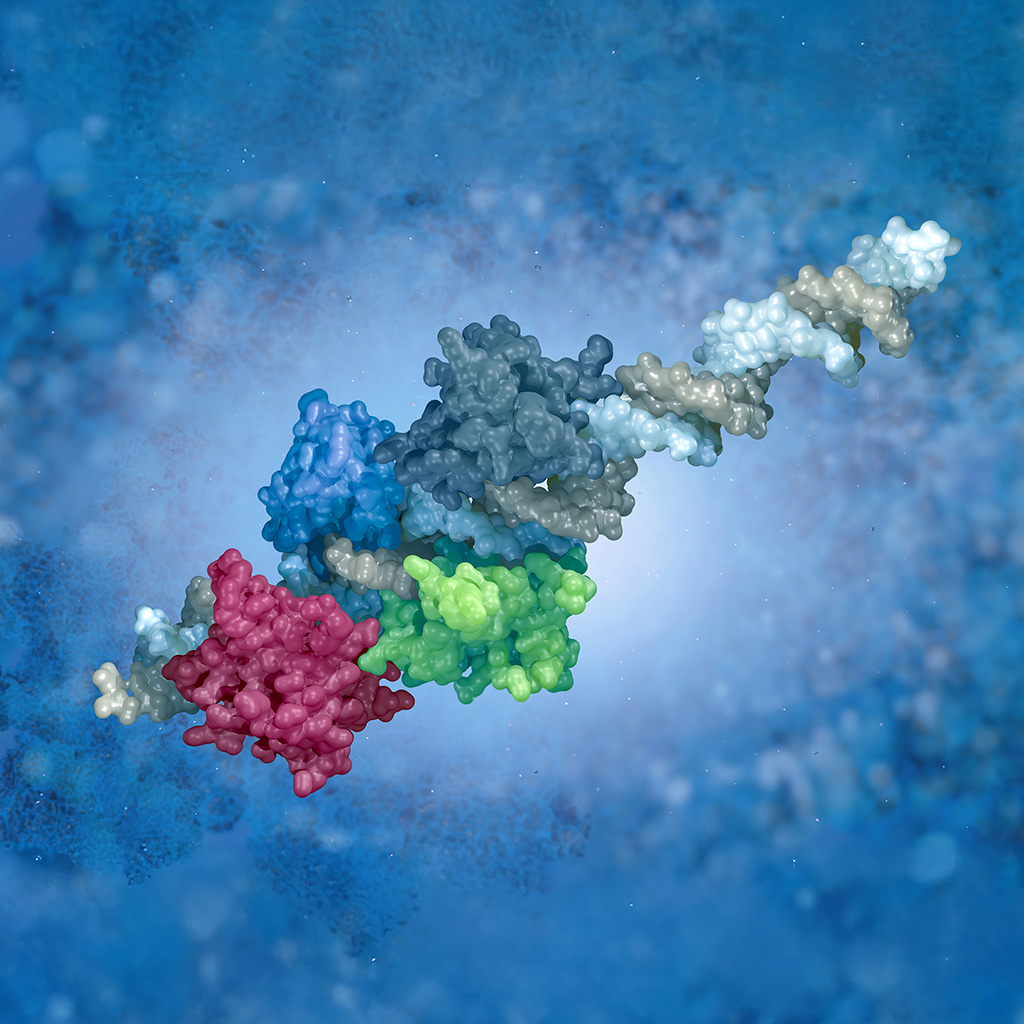
Read how Syngene’s partnership with a leading Biotech company working in the field of cancer therapy, grew from one small pilot project to over 100 FTEs today.






